Occurrence, Position and Isotopes of Hydrogen
Introduction:
Hydrogen, the most abundant element in the universe and the
third most abundant on the surface of the globe. Hydrogen has the simplest
atomic structure among all the elements around us in Nature. In atomic form it
consists of only one proton and one
electron. However, in elemental form it exists as a diatomic (H2)
molecule and is called dihydrogen.
Occurrence:
Dihydrogen
is the most abundant element in the universe (70% of the total mass of the
universe) and is the principal element in the solar atmosphere. The giant
planets Jupiter and Saturn consist mostly of hydrogen. However, due to its
light nature it is much less abundant
(0.15% by mass) in atmosphere. In the combined form it constitutes 15.4% of
the earth's crust and the oceans. In the combined form besides in water, it
occurs in plant and animal tissues, carbohydrates, proteins, hydrides including
hydrocarbons and many other compounds.
·
It is the third
most abundant element on the surface of the globe.
·
On the earth it is the ninth element in abundance.
Position of Hydrogen:
Hydrogen
is the first element in the periodic table. The elements in the periodic table
are arranged according to their electronic configurations. Hydrogen has
electronic configuration 1s1. On one hand, its electronic
configuration is similar to the outer
electronic configuration (ns1) of alkali metals, which belong to
the first group of the periodic table. On the other hand, like halogens (with
ns2 np5 configuration belonging to the seventeenth group
of the periodic table), it is short by one electron to the corresponding noble
gas configuration, helium (1s2).
Position of Hydrogen in Periodic Table:
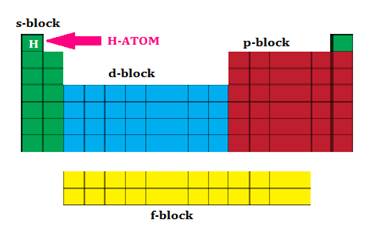
Hydrogen, therefore has
resemblance to alkali metals which lose one electron to form unipositive ions
as well as with halogens, which gain one
electron to form uninegative ion. Like alkali metals, hydrogen forms oxides, halides and sulphides. In fact, in terms of
ionization enthalpy, hydrogen resembles more with halogens. Like halogens, it
forms a diatomic molecule.
In terms of reactivity, it is very
low as compared to halogens. This is extremely small as compared to normal
atomic and ionic sizes of 50 to 200pm. As a consequence H+ does not exist freely and is always associated with
other atoms or molecules. Thus, it is unique
in behaviour and is therefore best placed separately in the periodic table.
It is placed on the left side on the
periodic table.
Isotopes of Hydrogen:
Isotopes
are the different atoms of the same element which have the same atomic number but diferent
mass number.
Occurrence of Isotopes:
·
The most abundant isotope of hydrogen is
protium.This in natural hydrogen to an extent of 99.4844%.
·
The remaining 0.0156% being deuterium.
·
Tritium being unstable because of its
radioactive nature.
·
The natural abundance of ![]() ,
,![]() are in the ratio of 1 : 1.56 ×
10-2 : 1 × 10-18.
are in the ratio of 1 : 1.56 ×
10-2 : 1 × 10-18.
Hydrogen has Three Isotopes:
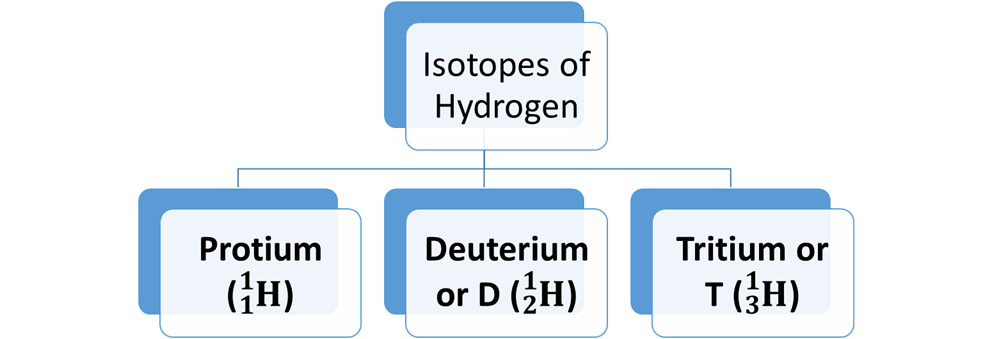
·
Protium (![]() )
)
·
Deuterium or D (![]() )
)
·
Tritium or T (![]() )
)
These isotopes differ from one
another in respect of the presence of
neutrons.
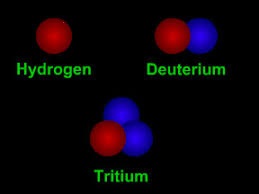
Ordinary hydrogen, protium, has no neutrons, deuterium (also known as
heavy hydrogen) has one and tritium has two neutrons in the nucleus. Hydrogen without any neutron is protium. Hydrogen
with one neutron is deuterium.
Hydrogen with two neutrons is tritium.
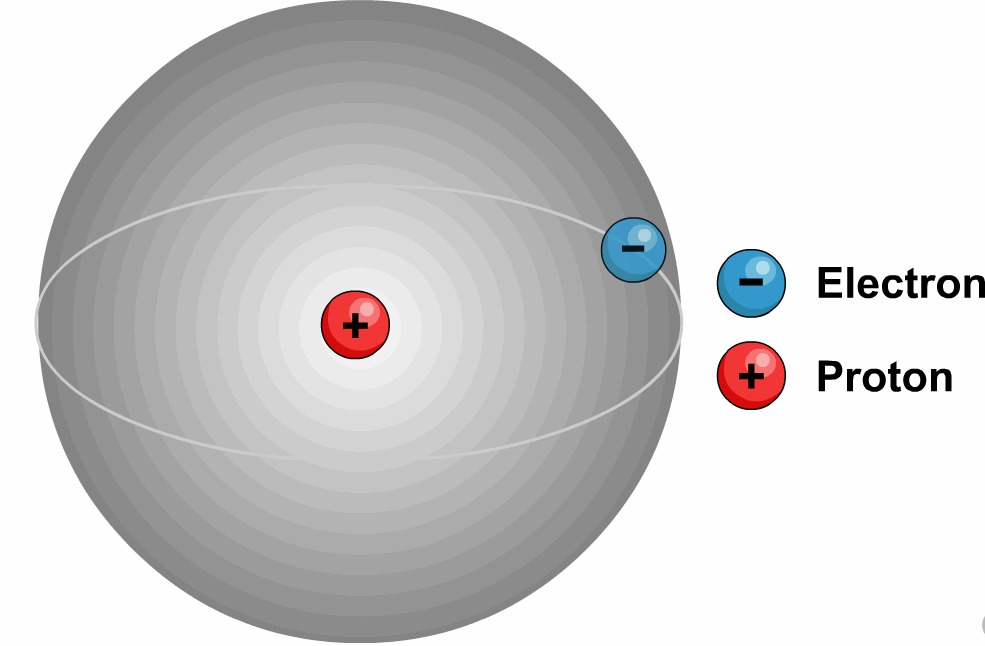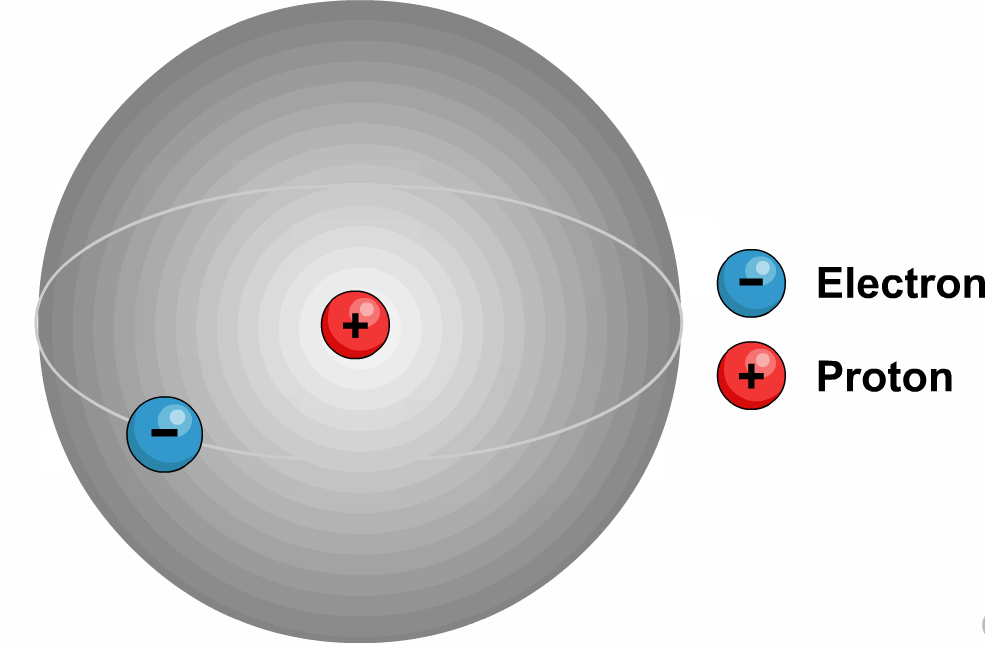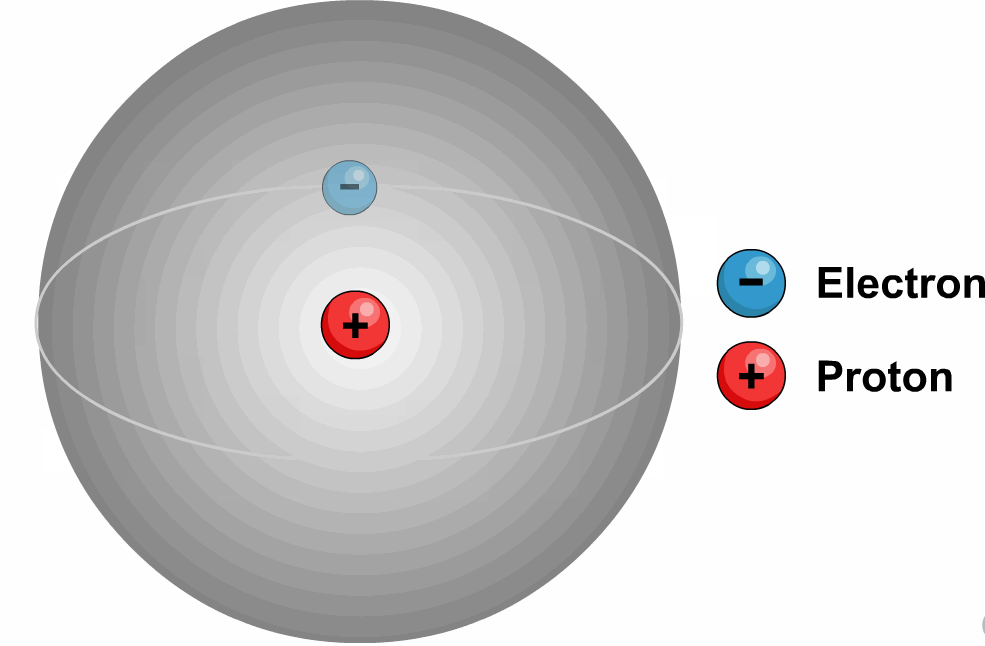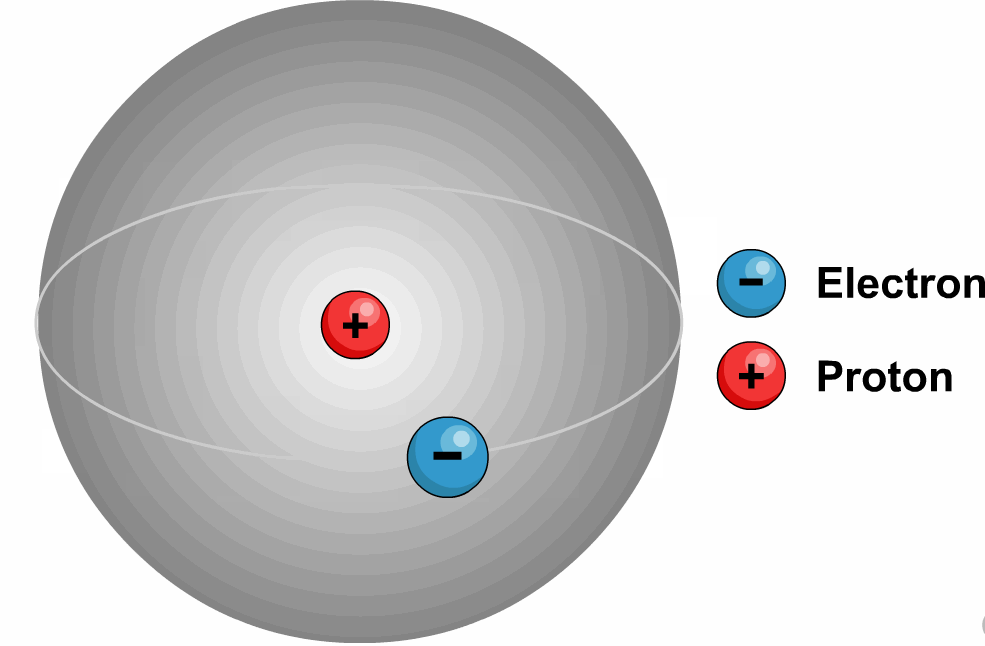
1. Protium or Ordinary Hydrogen (![]() ):
):
·
Protium, in
physics and chemistry means hydrogen-1 the most common isotope of the element hydrogen.
·
Its atomic number =mass number=1 and its
exact mass is 1.007825 amu.
·
It is a nonradioactive element.
·
It has only one electron, one proton and zero neutron.

2. Deutrium or Heavy Hydrogen (![]() or D):
or D):
·
Deuterium is a hydrogen
isotope consisting of one proton, one neutron and one electron.
·
Deuterium is roughly twice the mass of
protium (deuterium has a mass of 2.014102 amu).
·
It is a radioactive element.
·
Chemically, deuterium behaves similarly
to ordinary hydrogen (protium), but there are differences in bond energy and
length.
Deutrium
3. Tritium (![]() or T):
or T):
·
Its nucleus, consisting of one proton,
one electron and
two neutrons, has triple the mass of the nucleus of ordinary hydrogen.
·
Tritium is
a beta-emitting radioactive isotope of hydrogen.
·
It has an atomic mass of
3.0160492 u.
·
It has an atomic mass of
14.
|
Protium |
Deutrium |
Tritium |
|
1 proton, 1
electron, 0 neutron. |
1 proton, 1electron, 1 neutron. |
1 electron, 1proton, 2 neutron. |
|
Non-radioactive
element. |
Radioactive element. |
Radioactive element. |
|
Common isotope of
hydrogen. |
Stable isotope of hydrogen. |
Radioactive isotope of hydrogen. |