Preparation of Dihydrogen
Molecular hydrogen, sometimes called dihydrogen, is a diatomic molecule that is composed of two hydrogen atoms
held together by a covalent bond with the chemical formula H2.
Preparation
of Dihydrogen:
Dihydrogen may be obtained from water by reduction either with active
metals or by electricity. It can be prepared from three sources:
1. from water
2. from alkalis
3. from acids
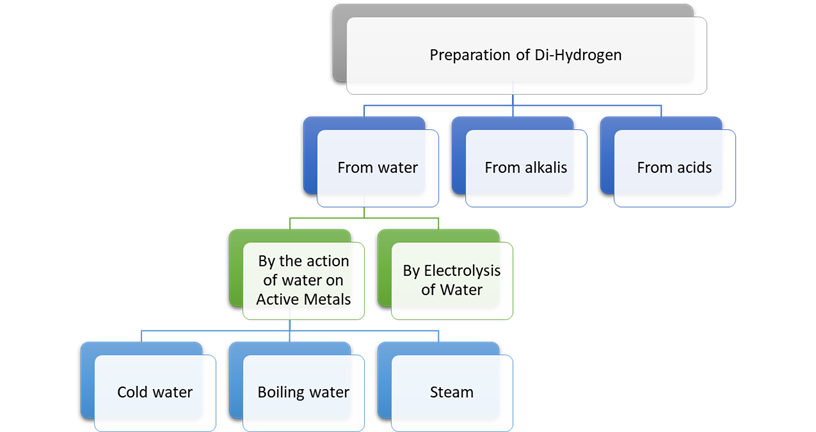
1. From Water:
a) By the
Action of Water on Active Metals:
·
Cold Water: Very active metals i.e. alkali and certain alkaline earth metals like
Na, K , Ca react with water at room temperature evolving dihydrogen.
The reaction with
alkali metal is so vigorous and exothermic that the hydrogen involved catches
fire. To slow down the reaction, amalgams of these metals are generally used.
In amalgams, only a
small surface area of the metal comes in contact with water and therefore the
reaction is slowed down.
·
Boiling water: Less active metals like Zn, Mg, Al decompose boiling water liberating dihydrogen.
|
Zn + H2O |
→ |
ZnO + H2 |
|
Mg + H2O |
→ |
MgO + H2 |
|
2Al + 3 H2O |
→ |
Al2O3+
H2 |
·
Steam: Still less active metals like Fe, Sn, Ni
decompose steam at high temperature (1023-1073K) evolving dihydrogen.
|
3Fe + 4H2O → Fe3O4 +
H2 |
b) By
Electrolysis of Water:
Dihydrogen of high purity is usually obtained by the electrolysis of
water in the presence of small amount of an acid or a base. During
electrolysis, dihydrogen is collected at cathode while dioxygen is liberated at
anode.
|
2H2O (l)
→ 2H2 (g) + O2 (g) |
Pure water is only
weakly ionized and hence is a poor conductor of electricity but presence of an
acid or a base makes it a better conductor of electricity.
This method is not
commercially used since it is quite expensive.
2. From Alkalis:
Metals like Be, Zn, Sn react with
boiling alkali solution liberating dihydrogen.
|
Zn + 2NaOH |
→ |
Na2ZnO2 + H2 |
|
Be + 2NaOH |
→ |
Na2BeO2 + H2 |
|
Sn + 2NaOH
+ H2O |
→ |
Na2SnO3 + H2 |
|
2Al + 2NaOH
+ 2 H2O |
→ |
2NaAlO2 + 3H2 |
3. From Acids:
Metals which are more electropositive than hydrogen (lie above hydrogen
in the electrochemical series) such as Zn, Fe, Mg
react with dilute mineral acids to liberate dihydrogen gas.
|
Zn + H2SO4 |
→ |
ZnSO4 + H2 |
|
Fe +2HCl |
→ |
FeCl2 + H2 |
Metals like
Copper, silver, Mercury which are less electropositive than hydrogen (lie below
hydrogen in the electrochemical series) do not liberate hydrogen from acids.
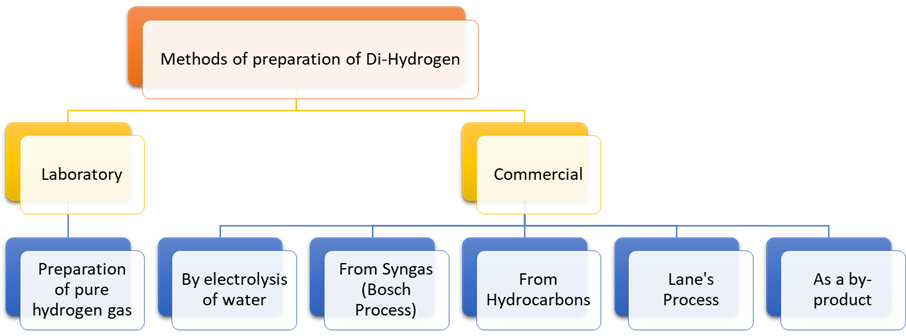
There
are two methods in preparation of dihydrogen:
1. Laboratory preparation
2. Commercial preparation
1. Laboratory Preparation:

In laboratory, dihydrogen is
prepared by reaction of dil H2SO4 on
granulated zinc.
|
Zn + H2SO4 → ZnSO4 +
H2 |
Granulated pieces of
zinc are placed in a Woulfe’s bottle and are covered
with water. The bottle is fitted with a thistle funnel and a delivery tube.
Conc.H2SO4 is poured slowly through the
thistle funnel. As the acid falls in the Woulfe’s bottle,it gets diluted and then
reacts with zinc evolving dihydrogen gas. It is collected by downward
displacement of water.
Sometimes the bubbles of dihydrogen produced stick to the surface of the
zinc metal preventing the further reaction of the acid on the metal. Such a
situation can be avoided by adding few crystal of copper sulphate to the
reaction mixture.
Preparation
of Pure Dihydrogen Gas:
·
By the action of pure
sulphuric acid on magnesium ribbon
|
Mg + H2SO4 (dil) → MgSO4 + H2 |
·
By the electrolysis
of a warm solution of barium hydroxide using platinum or Nickel electrodes
·
By the action of
water on sodium hydride
|
NaH + H2O
→ NaOH + H2 |
·
By the action of KOH
on scrap aluminium
|
2Al + 2KOH + 2H2O → 2KAlO2 +
3H2 |
2. Commercial
Preparation: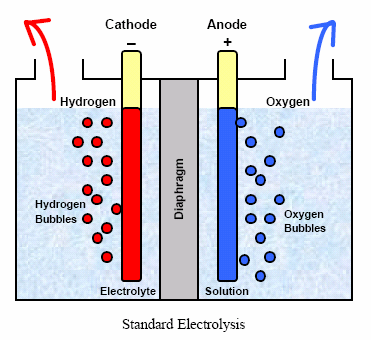
· By the Electrolysis of Water:
A small quantity of
acid or alkali is added to water to make it a good conductor and electrolysed
in a cell. In this cell, iron sheet is used as a cathode while nickel plated
iron sheet act as anode.
The two electrodes are separated from each other by an asbestos diaphragm which prevents
mixing of dihydrogen and dioxygen. On passing electric current, dihydrogen gas
is collected at cathode while dioxygen at anode.
When 20% NaOH solution is used for electrolysis, the decomposition
of water takes place as follows:
At cathode,
|
H+ +
e‾ |
→ |
H |
|
H+ +
H+ |
→ |
H2 |
At anode,
|
4OH‾ |
→ |
4OH + e‾ |
|
4OH‾ |
→ |
2H2O
+ O2 |
· From Syngas
(Bosch process):
When superheated steam is passed
over red hot coke or coal at 1270K in the presence of nickel catalyst, a
mixture of carbon monoxide and dihydrogen is produced.
|
C (s) + H2O
(g) → CO + H2 |
A mixture 1:1 of CO
and H2 was called water gas. All mixtures of CO and H2irrespective
of their composition are called synthetic gas or syngas.
This process of
producing syngas from coke or coal is coal gasification.
Syngas is produced
from sewage, sawdust, scrap wood, newspaper.
It is difficult to obtain pure hydrogen from water gas or Syngas, since
CO is difficult to remove. To remove CO and increase the production of
dihydrogen from syngas, CO of the syngas is oxidised to CO2 by
mixing it with more steam at 673 K in presence of iron chromate as catalyst.
|
CO (g) + H2O
(g) + H2O (g) → CO2 (g) + 2H2 (g) |
CO2 thus produced is removed either by scrubbing the
mixture with sodium arsenide solution or bypassing the mixture through water
under 30 atm pressure when carbon dioxide dissolves
leaving behind dihydrogen which is collected.
· From
Hydrocarbons:
Partial Oxidation of
Hydrocarbons: A mixture of hydrocarbons is mixed with steam and
passed over heated Nickel catalyst at 1270 K.
|
CnH2n+2 +
nH2O → nCO + (2n+1) H2 |
Natural gas may also
be used.
|
CH4 (g) + H2 (g) |
Whole process of
obtaining dihydrogen from natural gas is called steam
reforming process.
Thermal Cracking of Natural Gas: Dihydrogen may also be obtained by thermal cracking of natural gas at
1270 K in the presence of a catalyst.
|
CH4 → C + 2 H2 |
· Lane’s
Process:
Dihyrogen
can also be manufactured by passing alternate currents of steam and water gas
over red hot iron. It consists of two stages:
Oxidation Stage: Super heated steam is passed over iron filling heated to about 1023 – 1073 K when
hydrogen is formed and magnetic oxide of iron is left behind.
|
3Fe + 4 H2O
→ Fe3O4 + 4H2 |
Reduction stage: When the whole of iron has being
oxidised, the steam supply is cut off and a steam of water gas is passed to
reduce Fe3O4 back to iron.
|
Fe3O4
+ 4H2 |
→ |
3Fe + 4H2O |
|
Fe3O4
+ 4CO |
→ |
3Fe + 4CO2 |
By passing steam and
water gas alternatively over heated iron, dihydrogen gas can be manufactured
from a small quantity of iron.
· As a By –
Product:
Large quantities of dihydrogen are
obtained as a by-product in various industries.
1) From petroleum
cracking plants
2) In the manufacture
of sodium hydroxide and chlorine by electrolysis of brine solution.
At anode,
|
2Cl‾ (aq) → Cl2
(g) + 2e‾ |
At Cathode,
|
2H2O
(l) + 2e‾ → H2 (g) + OH‾ (aq) |
Overall
reaction:
|
2Cl‾ (aq)
+ 2H2O (l) → Cl2 (g) + H2 (g) + OH‾ (aq) |