Physical and Chemical
Properties of Water
Water:
A major
part of all living organisms is made up of water. Human body has about 65% and
some plants have as much as 95% water. It is a crucial compound for the
survival of all life forms. It is a solvent
of great importance. The distribution of water over the earths surface is not
uniform.
Estimated World Water Supply:
|
Source |
% of Total |
|
Lakes |
0.009 |
|
Oceans |
97.33 |
|
Saline
lakes and inland seas |
0.008 |
|
Polar
ice and glaciers |
2.04 |
|
Ground
water |
0.61 |
|
Soil
moisture |
0.005 |
|
Atmospheric
water vapour |
0.001 |
|
Rivers |
0.0001 |
Physical Properties of Water:
·
It is a colourless
and tasteless liquid.
·
The unusual properties of water in the condensed
phase (liquid and solid states) are due to the presence of extensive hydrogen
bonding between water molecules. This leads to high freezing point, high boiling point, high heat of vaporisation and high heat of fusion.
·
In comparison to other liquids water has a higher
specific heat, thermal conductivity, surface tension, dipole moment and
dielectric constant, etc.
·
These properties allow water to play a key role in
the biosphere.
Physical Properties of H2O:
|
Property |
H2O |
|
Molecular mass (g mol1) |
18.0151 |
|
Melting point/K |
273.0 |
|
Boiling point/K |
373.0 |
|
Enthalpy of formation/kJ mol1 |
-285.9 |
|
Enthalpy of vaporisation (373K)/kJ mol1 |
40.66 |
|
Enthalpy of fusion/kJ mol1 |
6.01 |
|
Temp of max. density/K |
276.98 |
|
Density (298K)/g cm-3 |
1.0000 |
|
Dielectric
constant/C2 /Nm2 |
78.39 |
|
Viscosity/centipoise |
0.8903 |
|
Electrical conductivity (293K/ohm1 cm1)
|
5.7108 |
Structure of Water:
Water
has a very simple atomic structure. This structure consists of two hydrogen
atoms bonded to one oxygen atom. The nature of the atomic structure of water
causes its molecules to have unique electrochemical properties. The hydrogen
side of the water molecule has a slight positive charge. On the other side of
the molecule a negative charge exists. The atomic structure of a water molecule
consists of two hydrogen H atoms joined to one oxygen O atom.
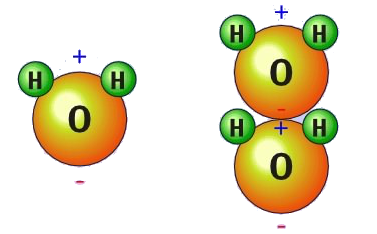
Structure of Ice:
Ice has a highly ordered
three dimensional hydrogen bonded structure.
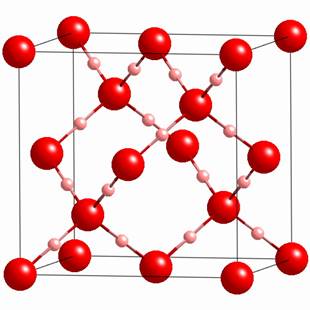
Chemical Properties of Water:
Water reacts with a large number of
substances. Some of the important reactions are given below:
1. Amphoteric Nature:
It
has the ability to act as an acid as well as a base i.e., it behaves as an amphoteric
substance. In the Bronsted sense it acts as an acid
with NH3 and a base with H2S.
|
H2O
(l) + NH3 (aq) |
|
OH-(aq) + |
|
H2O
(l) + H2S (aq) |
|
H3O+
(aq) + HS-
(aq) |
The auto-protolysis
(self-ionization) of water takes place as follows:
|
H2O (l) + H2O (l)
|
2. Redox Reactions Involving
Water:
·
Water can be easily reduced to dihydrogen by
highly electropositive metals.
|
2H2O (l) + 2Na (s) |
·
Thus, it is a great source of dihydrogen. Water is
oxidised to O2 during photosynthesis.
|
6CO2 (g)
+ 2H2O (l) |
·
With fluorine also it is oxidised to O2.
|
2F2 (g) + 2H2O (l)
|
3. Hydrolysis Reaction:
Due to
high dielectric constant, it has a very strong hydrating tendency. It dissolves
many ionic compounds. However, certain covalent and some ionic compounds are
hydrolysed in water.
|
P4O10 (s) + 6H2O (l) |
4. Hydrates Formation:
From
aqueous solutions many salts can be crystallised as hydrated salts. Such an
association of water is of different types:
a)
Coordinated water
b)
Interstitial water
c)
Hydrogen-bonded water.