Classification of Solid State

Amorphous
and Crystalline Solids
On
the basis of the nature of order present in the arrangement of their
constituent particles, Solids can be classified as:
·
Amorphous Solid
·
Crystalline Solids
Crystalline
Solids
A crystalline solid usually consists of a
large number of small crystals, each of them having a definite characteristic
geometrical shape. The arrangement of constituent particles (atoms, molecules
or ions) in a crystal is ordered and repetitive in three dimensions.
Crystal has a long range order
which means that there is a regular pattern of arrangement of particles which
repeats itself periodically over the entire crystal. Sodium
chloride and quartz are typical examples of crystalline solids.
Amorphous
Solid
Glass, rubber and many plastics do not form
crystals when their liquids solidify on cooling. These are called amorphous
solids. The term amorphous comes from
the Greek word amorphos, meaning no form. The
arrangement of constituent particles (atoms, molecules or ions) in such a solid
has only short range order.
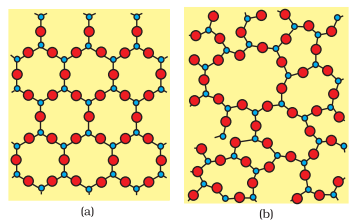
Two dimensional structure of
(a) quartz and (b) quartz glass
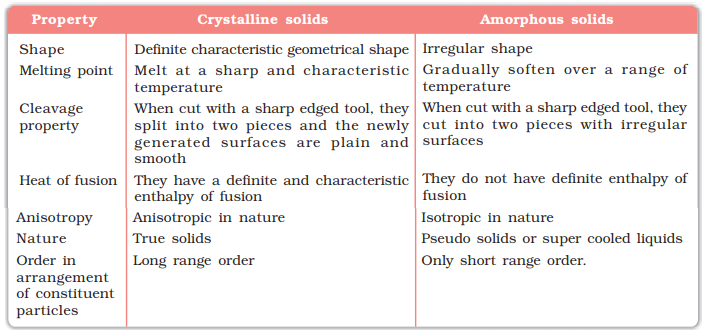
Distinction
between Crystalline and Amorphous Solids
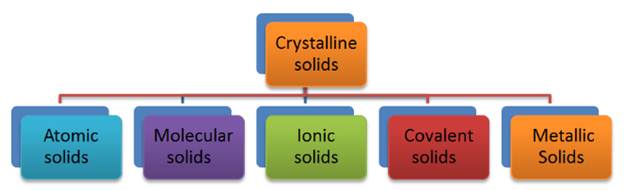
Classification of Crystalline Solid
We will classify crystalline
solids on the basis of nature of intermolecular forces or bonds that hold the
constituent particles together. These are — (i) Van
der waals forces; (ii) Ionic bonds; (iii) Covalent
bonds; and (iv) Metallic bonds. On this basis,
crystalline solids are classified into four categories viz.,
·
Molecular solids
·
Ionic solids
·
Metallic solids
·
Covalent solids
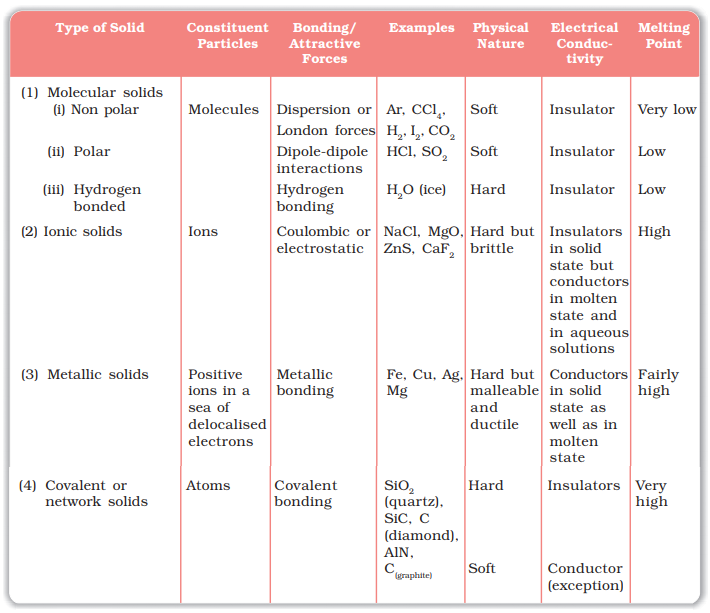
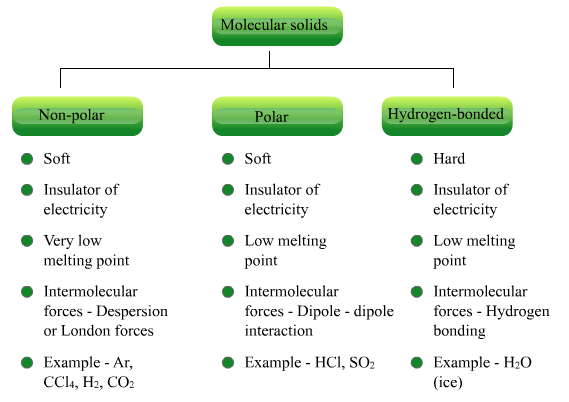
Molecular Solids
Molecules are the constituent
particles of molecular solids. These are further sub divided into the following
categories:
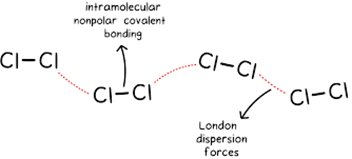
(i) Non polar Molecular Solids:
Ø They comprise of either
atoms, for example, argon and helium or the molecules formed
by non polar covalent bonds.
Ø
Example: H2, Cl2 and I2.
Ø In these solids, the atoms or molecules are held by weak dispersion forces or London forces.
Ø These
solids are soft and non-conductors of electricity.
Ø They
have low melting points and are usually in liquid or gaseous state at room
temperature and pressure.
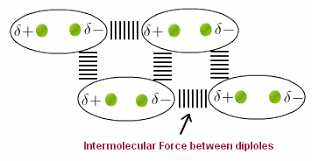
(ii) Polar Molecular Solids:
Ø The molecules of substances
like HCl, SO2,
etc., are
formed by polar covalent bonds.
Ø The molecules in
such solids are held together by relatively stronger
dipole-dipole interactions.
Ø These solids are soft and non-conductors of electricity.
Ø Their
melting points are higher than those of non polar molecular
solids yet most of these are gases or liquids under room temperature and
pressure.
Ø Example:
Solid SO2 and solid NH3.
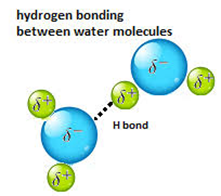
(iii) Hydrogen Bonded Molecular Solids:
Ø
The molecules of
such solids contain polar covalent bonds between H and F, O or N
atoms.
Ø Strong hydrogen bonding binds molecules of such solids like H2O (ice).
Ø They
are non-conductors of electricity.
Ø Generally
they are volatile liquids or soft solids under room temperature and pressure.
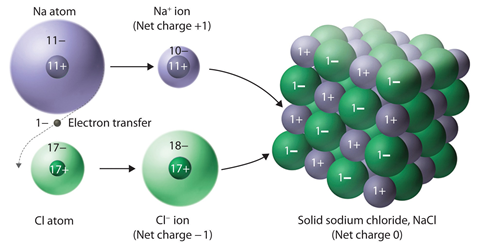
Ionic Solids
Ions are the constituent
particles of ionic solids. Such solids are formed by the three dimensional arrangements
of cations and anions bound by strong coulombic (electrostatic)
forces.
Characteristics of
Ionic Solids
Ø They
are hard, brittle and have low volatility.
Ø They
have high melting points.
Ø They
are poor conductors of electricity in solid state, however they become good
conductors of electricity in molten state or in dissolved state.
Ø They
are generally soluble in polar solvents like water.
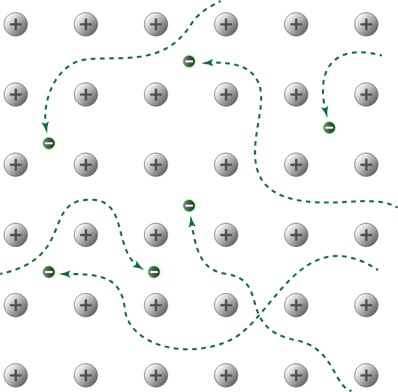
Metallic Solids
Metallic solids
are solids composed of metal atoms that are held together by metallic
bonds. These bonds are like huge molecular orbitals that span
across the whole solid. This means the electrons in metallic solids
are delocalized.
Characteristics of
Metallic Solids
Ø They
generally range from soft to very hard.
Ø They
are malleable and ductile.
Ø They
are good conductors of heat and electricity.
Ø They
possess bright lustre.
Ø They
have high melting and boiling points.
Ø They
have moderate heats of fusion.
Covalent or Network Solids
It
is a chemical compound (or element) in which the atoms are bonded
by covalent bonds in a continuous network extending throughout
the material. In a network solid there are no
individual molecules, and the entire crystal or amorphous
solid may be considered a macromolecule.

Characteristics of
Covalent Solids
Ø They
are very hard.
Ø Diamond
and silicon carbide are typical examples of such solids.
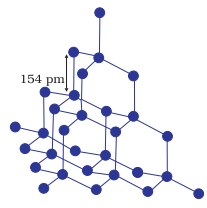
Network structure of diamond
Ø Although
Graphite also belongs to this class of crystals, but it is soft and is a
conductor of electricity. Its exceptional properties are due to its typical
structure.
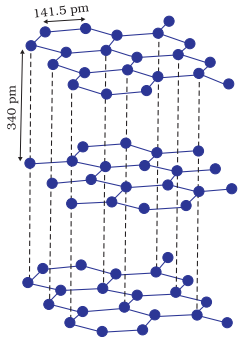
Structure
of graphite
Ø They
have very high melting points.
Ø They
are poor conductors of heat and electricity.
Ø They
have high heats of fusion.
Different
properties of the four types of solids