Electrical
and Magnetic Properties
Electrical Properties
Electrical
conductivity of solids may arise through the motion of electrons and positive
holes (electronic conductivity) or through the motions of ions (ionic
conductivity).
The conduction
through electrons is called n-type conduction and through positive holes is
called p – types conduction.
Electrical
conductivity of metal is due to motion of electrons and it increases with the
number of electrons available to participate in the conduction process.
Pure
ionic solids where conduction can take place only through motion of ions are
insulators. However, the presence of defects in the crystal structure increases
their conductivity.
On the basis of electrical
conductivity the solids can be classified into three types –
·
Conductors
·
Insulators
·
Semi
conductors
Metal (Conductors):
They
allow the maximum portion of the applied electric field to flow through them
and have conductivities in order of 106 – 108 ohm-1.
Insulators:
They
have low conductivities i.e. they do not practically allow the electric circuit
to flow through them. The electrical conductivity is in order 10-10
– 10-20 ohm-1 m-1
Semi conductors:
The
solids with intermediate conductivities at the room temperature. Semi conductors
allow a portion of electric current to flow through them.
Actually
semi conductors are those solids which are perfect insulators at absolute zero,
but conduct electric current at room temperature.
Conduction of Electricity in Metals
A
conductor may conduct electricity through movement of electrons or ions.
Metallic conductors belong to the former category and electrolytes to the
latter.
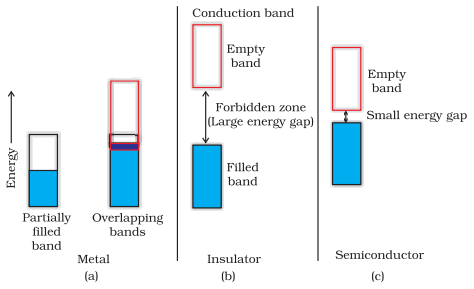
Metals
conduct electricity in solid as well as molten state. The conductivity of
metals depend upon the number of valence electrons available per atom. The
atomic orbitals of metal atoms form molecular orbitals which are so close in
energy to each other as to form a band. If this band is partially filled or it
overlaps with a higher energy unoccupied conduction band, then electrons can
flow easily under an applied electric field and the metal shows conductivity
(below figure a).
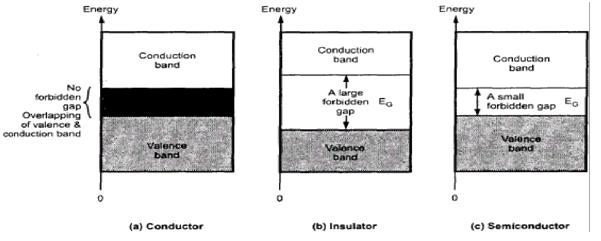
If the
gap between filled valence band and the next higher unoccupied band (conduction
band) is large, electrons cannot jump to it and such a substance has very small
conductivity and it behaves as an insulator (below figure b).
Distinction among (a) metals (b)
insulators and (c) semiconductors. In each case, an unshaded area represents a
conduction band.
Conduction of Electricity in Semiconductors
In case
of semiconductors, the gap between the valence band and conduction band is
small (above figure c). Therefore, some electrons may jump to conduction band
and show some conductivity. Electrical conductivity of semiconductors increases
with rise in temperature, since more electrons can jump to the conduction band.
Substances like silicon and germanium show this type of behaviour
and are called intrinsic semiconductors.
The
conductivity of these intrinsic semiconductors is too low to be of practical
use. Their conductivity is increased by adding an appropriate amount of
suitable impurity. This process is called doping. Doping can be done with an
impurity which is electron rich or electron deficient as compared to the
intrinsic semiconductor silicon or germanium. Such impurities introduce
electronic defects in them.
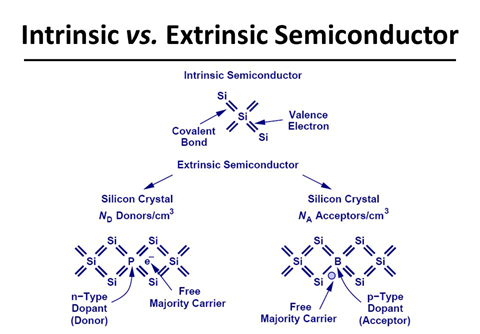
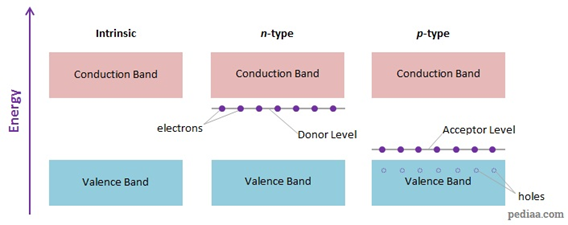
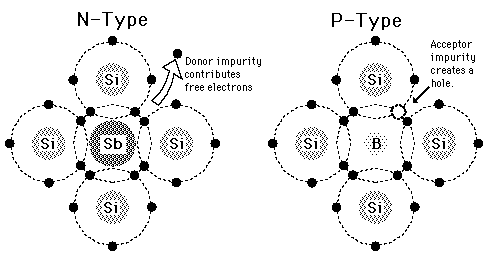
(i)
n-type semiconductors
Silicon doped
with 15 group elements like phosphorus is called ntype
semiconductor. The conductivity is due to the presence of negative charge
(electrons),
(ii)
p-type semiconductors
Silicon doped
with 13 group element like gallium is called p-type semiconductor. The
conductivity is due to the presence of positive holes.
·
Some typical 13-15 compounds are InSb, AlP and GaAs and SOme typical 12-16 compounds are ZnS, CdS. CdSe
and HgTe.
·
These exhibit electrical and optical properties of great use in
electronic industry. Magnetic Properties of Solids
Magnetic Properties
Ø The
magnetic properties of different materials are studies in terms of their
magnetic moments which arise due to the orbital motion and spinning motion of
the electron.
Ø As
electron is charged particle, the circular motion of the electric charge causes
the electron to act as a tiny electro magnet.
Ø The
magnetic moment of the magnetic field generated due to orbital motion of the
electron is along the axis of rotation.
Ø The
electron also possesses magnetic moment due to the spin which is directed along
the spin axis.
Ø Thus,
magnetic moment of the electron is due to travelling in closed path (orbital
motion) about the nucleus and spinning on its axis.
Ø For
each electron spin magnetic moment is ±μB where μB, Bohr Magneton is the fundamental unit of magnetic
moment and is equal to 9.27 × 10-24 em2.
Ø The
magnetic moment due to orbital motion is equal to Mlμ
B where Ml is the magnetic quantum number of the electron.
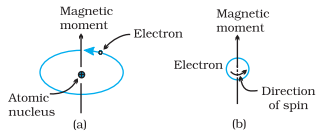
Demonstration of the magnetic moment
associated with (a) an orbiting electron and (b) a spinning electron
On
the basis of their magnetic properties, substances can be classified into five
categories:
i.
Paramagnetic
ii.
Diamagnetic
iii.
Ferromagnetic
iv.
Antiferromagnetic and
v.
Ferrimagnetic
Paramagnetic
Substances
These are
attracted by the magnetic field and have unpaired electrons. These lose
magnetism in the absence of magnetic field, e.g., O2, Cu2+,
Fe3+, etc.
Diamagnetic
Substances
These are weakly
repelled by the magnetic field and do not have any unpaired electron, e.g., TiO2,
V2O5, C6H6, NaCI,
etc.
Ferromagnetic
Substances
These are
attracted by the magnetic field and show permanent magnetism even ill the
absence of magnetic field e.g., Fe, Co and Ni.
Anti-ferromagnetic
Substances
These substances
have net magnetic moment zero due to compensatory alignment of magnetic
moments, e.g., MnO, MnO2, FeO, etc.
Ferrimagnetic
Substances
These substances
have a net dipole moment due to unequal parallel and anti-parallel alignment of
magnetic moments, e.g., Fe3O4, ferrites, etc.
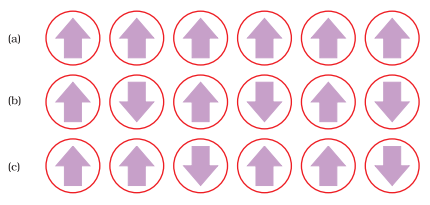
Schematic alignment
of magnetic moments in (a) ferromagnetic (b) antiferromagnetic and (c) ferromagnetic
|
Properties |
Description |
Alignment of Magnetic
Dipoles |
Examples |
Applications |
|
Diamagnetic |
Feebly repelled by the
magnetic fields. Non-metallic elements (excepts O2, S) inert gases and
species with paired electrons are diamagnetic |
All paired electrons |
TiO2, V2O5, NaCl, C6H6 (benzene) |
Insulator |
|
Paramagnetic |
Attracted by the magnetic field due to the presence of
permanent magnetic dipoles (unpaired electrons). In magnetic field, these
tend to orient themselves parallel to the direction of the field and thus,
produce magnetism in the substances. |
At least one unpaired electron |
O2,Cu2+,Fe3+,TiO, Ti2O3,VO,VO2 , CuO |
Electronic appliances |
|
Ferromagnetic |
Permanent magnetism
even in the absence of magnetic field, Above a temperature called Curie
temperature, there is no ferromagnetism. |
Dipoles are aligned in
the same direction |
Fe, Ni, Co, CrO2 |
CrO2 is used in audio
and video tapes |
|
Antiferromagnetic |
This arises when the dipole alignment is zero due to
equal and opposite alignment. |
|
MnO, MnO2, Mn2O, FeO, Fe2O3; NiO,
Cr2O3, CoO, Co3O4, |
|
|
Ferrimagnetic |
This arises when there
is net dipole moment |
|
Fe3O4, ferrites |