Imperfections in Solids
In a crystalline
solid, the atoms, ions and molecules are arranged in a definite repeating
pattern, but some defects may occur in the pattern. Derivations from perfect
arrangement may occur due to rapid cooling or presence of additional particles.
The defects are of two types, namely point
defects and line defects
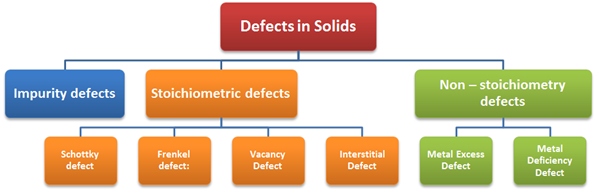
Point
Defects:
Point defects are
the irregularities or deviations from ideal arrangement around a point or an
atom in a crystalline substance Point defects can be classified into three
types:
·
Stoichiometric defects
·
Impurity defects
·
Non–stoichiometric defects
Stoichiometric
Defect
These are point
defects that do not disturb the stoichiometric of the solid. They are also
called intrinsic or thermodynamic defects.
In non-ionic
solids, these are of two types,
·
Vacancy
Defect
·
Interstitial
Defect
In ionic solids,
these are of two types,
·
Frankel defect
·
Schottky defect
Vacancy Defect:
When
some of the lattice sites are vacant, the crystal is said to have vacancy
defect. This results in decrease in density of the substance. This defect can
also develop when a substance is heated.
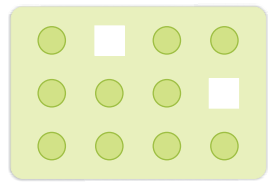
Vacancy
defects
Interstitial
Defect:
When
some constituent particles (atoms or molecules) occupy an interstitial site,
the crystal is said to have interstitial defect. This defect increases the
density of the substance.
Frenkel Defect:
This
defect is shown by ionic solids. The smaller ion (usually cation) is dislocated
from its normal site to an interstitial site. It creates a vacancy defect at
its original site and an interstitial defect at its new location.
Frenkel
defect is also called dislocation defect. It does not change the density of the
solid. Frenkel defect is shown by ionic substance in
which there is a large difference in the size of ions, for example, ZnS, AgCl, AgBr
and AgI due to small size of Zn2+ and Ag+
ions.

Frenkel
Defect
Schottky Defect:
It is
basically a vacancy defect in ionic solids. In order to maintain electrical
neutrality, the number of missing cations and anions are equal.
Like
simple vacancy defect, Schottky defect also decreases
the density of the substance. Number of such defects in ionic solids is quite
significant. For example, in NaCl
there are approximately 106 Schottky pairs
per cm3 at room temperature. In 1 cm3 there are about 1022
ions. Thus, there is one Schottky defect per 1016
ions. Schottky defect is shown by ionic substances in
which the cation and anion are of almost similar sizes. For example, NaCl, KCl, CsCl
and AgBr.
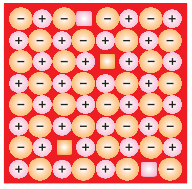
Schottky
Defect
Impurity
Defect
It arises when
foreign atoms or ions aloe present in the lattice. In case of ionic compounds,
the impurity 1S also ionic in nature. When the impurity has the same charge as
the host ion. It just substitutes some of the host ions.
Impurity defects
can also be introduced by adding impurity ions having different charge than
host ions. E.g., molten NaCl containing a little
amount of SrCl2 is crystallised. In such
cases,
Cationic vacancies produced = [number of
cations of higher valence × Difference in valence of the host cation and cation
of higher valence]
Non-Stoichiometric
Defect
Non-stoichiometric crystals are those
which do not obey the law of constant proportions.
The numbers of positive and negative ions
present in such compounds are different from those expected from their ideal
chemical formulae. However, the crystal as a whole in neutral. These
defects are of two types:
·
Metal excess defect
·
Metal deficiency defect
Metal
excess defect
Metal excess
defect due to anionic vacancies: Alkyl halides like NaCl
and KCl show this type of defect.
When crystals of NaCl are heated in
an atmosphere of sodium vapour, the sodium atoms are
deposited on the surface of the crystal. The Cl– ions diffuse to the
surface of the crystal and combine with Na atoms to give NaCl.
This happens by loss of electron by sodium atoms to form Na+ ions.
The released electrons diffuse into the crystal and occupy anionic sites. As a
result the crystal now has an excess of sodium.
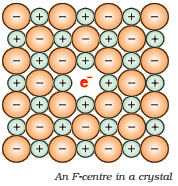
The anionic sites occupied by unpaired electrons are called F-centres (from the German word Farbenzenter
for colour centre). They
impart yellow colour to the crystals of NaCl. The colour results by
excitation of these electrons when they absorb energy from the visible light
falling on the crystals.
Metal deficiency
defect due to cation vacancy: Metal excess
defect due to presence of extra cations at interstitial sites, e.g., zinc oxide
is white in colour at room temperature. On beating,
it loses oxygen and turns yellow.
![]()
Now
there is excess of zinc in the crystal and its formula becomes Zn1+xO.
The excess Zn2+ ions move to interstitial sites and the electrons to
neighbouring interstitial sites.
Metal deficiency defect
·
It is due to the absence of a metal ion from its
lattice site and charge is balanced by ion having higher positive charge.
·
Transition metals exhibit this defect, e.g., FeO, which is found in the composition range from Fe0.93O
to Fe0.96O.
·
In crystal of FeO, some
Fe2+ cations are missing and the loss of positive charge is made up
by the presence of required number of Fe3+ ions.