Mechanism of Substitution Reactions
Nucleophilic substitution
reactions are of two types
(a) SN1 type
Unimolecular nucleophilic
reactions proceed in two steps:
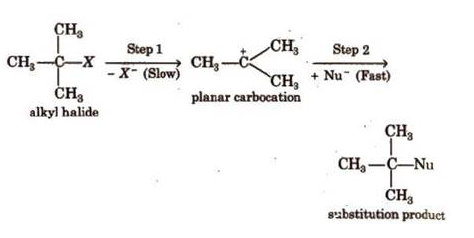
Rate, r = k [RX). It is a first
order reaction.
Reactivity order of alkyl
halide towards SN1 mechanism
3° >
2° > 1°
Polar
solvents, low concentration of nucleophiles and weak nucleophiles favour SN1 mechanism.
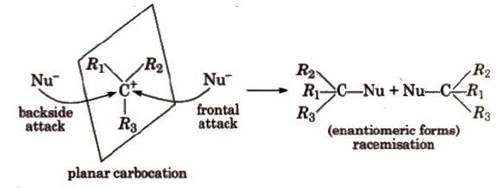
In SN1
reactions, partial racemisation occurs due to the
possibility of frontal as well as backside attack on planar carbocation.
(b) SN2 type (Bimolecular nucleophilic
substitution)
These
reactions proceed in one step and is a second order reaction with r = k[RX] [Nu].
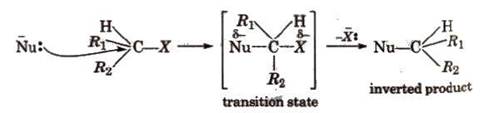
During
SN2
reaction, inversion of configuration occurs (Walden inversion) i.e., starting
with dextrorotatory halide a laevo product is
obtained and vice-versa, e.g.,
Reactivity of halides towards SN2
mechanism is
1° >
2° > 3°
Rate of
reaction in SN2
mechanism depends on the strength of the attacking nucleophile. Strength of
some common nucleophiles is
:CN- > : I- > : OR- > : OH- > CH3COO:
> H2O
> F-
Non-polar
solvents, strong nucleophiles and high concentration of nucleophiles favour SN2 mechanism.
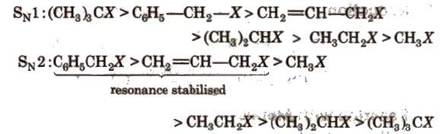
Relative rates of some alkyl
halides in SN1
and SN2
reactions are in the order
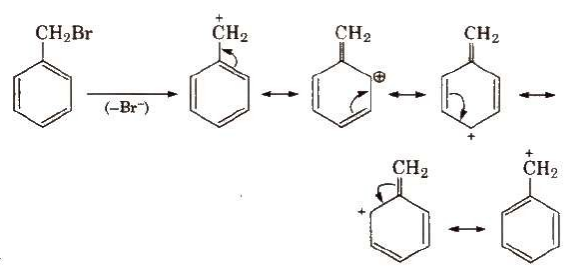
Resonating structure of benzyl carbocations are
Relative reactivity of alkyl
halides for same alkyl group is
RI >RBr> RCI > RF