Optical Isomerism
Compounds (with same molecular formula) that
differ in the way they rotate the plane polarized light.
·
Plane
polarized light − when ordinary light is passed through a nicol
prism or a diffraction grating such that light of a single wavelength is obtained.
·
The instrument
used to generate plane polarized light from ordinary light is called polarimeter.
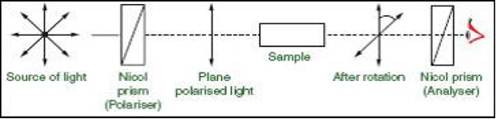
Polarimeter Set-Up:
It has a source of light from which when ordinary light
passes through the nicol prism, it generates plane polarized
light and when this plane polarized light passes through the sample containing
optically active compound, it rotates the light by some degree
Optically active compounds can rotate the
plane polarized light in
·
Clockwise (dextro-rotatory, (+)
d − form) or
·
Anti-clockwise (laevo-rotatory,
(−), l − form) direction.
·
The two forms of same compound, (+) and (−) forms
are called optical isomers.
·
When all 4 substituents of a carbon atom are
non-identical (different), then that molecule lacks symmetry. Such a carbon
atom is called asymmetric carbon atom or a stereo center.
·
When objects have non-supersuperimposable
mirror images, such an object is called chiral. For example, our hands are identical but
they cannot be superimposed. However, a symmetrical object like a sphere is
identical with its mirror image as well as super imposable on it. Hence, it is
an example of an achiral object.
·
In organic compounds, presence of an asymmetric carbon atom shows
that the molecule is chiral.
Example 1:
propan-2-ol (CH3)2CH − OH
·
We can determine if propan-2-ol is chiral if it has an
asymmetric carbon atom. However, the carbon bearing –OH group is attached to
two identical methyl groups such that all 4 substituents of the carbon atom are
not different. Thus, it is an achiral molecule.
·
We can also determine if its
chiral by obtaining its mirror image, rotating it by 180o and then superimposing it. If image (C) is
superimposable on (A), then it is achiral.
·
We can easily superimpose (C) on (A) to find that the
given molecule is achiral.
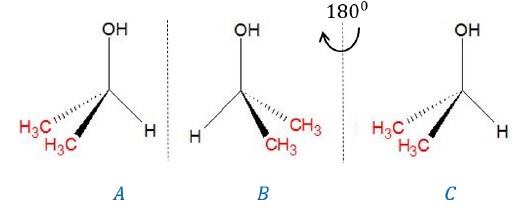
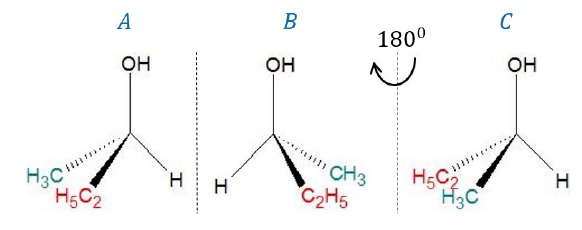
Example 2:
Butan-2-ol (CH3 − CH2 − CH(OH) −
CH3)
·
Carbon bearing the –OH group is attached to 3 different
groups (−H,−CH3,−C2H5)
such that it is an asymmetric carbon. Hence, the molecule is chiral.
·
By superimposing image (C) on (A) where (C) is obtained
after rotating the mirror image (B) of butan-2-ol by 180o, we can
also note that the images are not superimposable, again confirming that the
molecule is chiral.
The stereoisomers
which are non-superimposable mirror images of each other called enantiomers.
These possess
identical physical properties like melting point, boiling point, index of
refraction etc.
Only difference − rotation of plane polarised
light.
When a mixture
contains equal amount of both enantiomers, the net optical rotation is zero. It
is because the degree of clockwise rotation caused by the d − form is
cancelled exactly by anticlockwise rotation caused by l − form. This is
called racemic mixture.
The process of
converting an enantiomer into racemic mixture is called racemisation.
Retention of
Configuration − It is the
retention of spatial arrangement of groups of an asymmetric center during a
chemical reaction.
When two species
having same relative configuration can be correlated as XCabc
→ YCabc, there
is retention of configuration
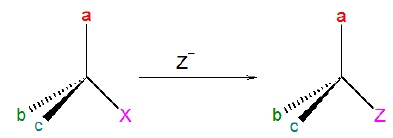
Condition for retention of configuration: No bond to
the stereocenter is broken.
For example: when
2 − methylbutan − 1 − ol is heated with conc. HCl.
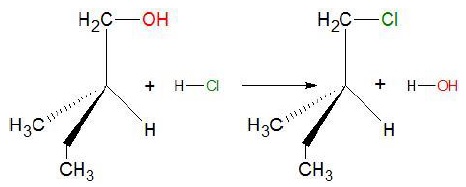
In the above
reaction, we can see that no bond to the stereocenter
or the asymmetric carbon atom is broken such that the configuration is
retained.
Inversion of
Configuration − It is the
loss of spatial arrangement of groups of an asymmetric center during a chemical
reaction.
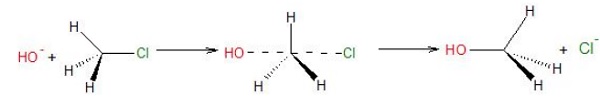
SN2
reactions involve inversion of configuration. As the nucleophile attacks the C −
X bond from back side, the configuration gets inverted like an umbrella
flipping upside down.
Optical Isomerism as a Part of Nuclephilic Reactions
SN1 Mechanism
Step 1 − (CH3)3C
− X ⇄
(CH3)3C+ + X−
Step 2 − OH− + (CH3)3C+ → (CH3)3C −
OH
·
The intermediate carbocation generated is planar.
·
Nucleophile can attack from above or below the plane such
that products are obtained with both retention and inversion of configuration.
·
If 50% products has inversion of configuration and
remaining 50% has retention of configuration, then the resulting mixture is a
racemic mixture.
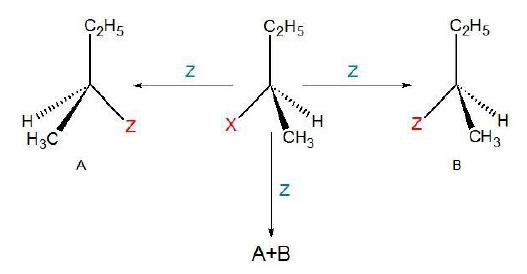
Example
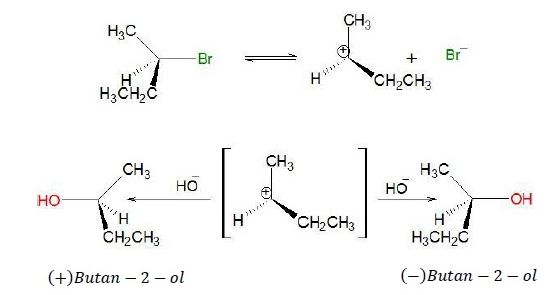
As can be seen
from the mechanism, (+)Butan
− 2 − ol has inverted configuration while
(−)Butan − 2 − ol
has retention of configuration with respected to the starting reactant.
Reaction at
asymmetric carbon can result in three configurations −
·
Retension − If only (B) is obtained.
·
Inversion − If only
(A) is obtained.
·
Racemisation − If both (A) and (B) are present in 50 : 50 ratio.