Physical and Chemical
Properties
Physical Properties of Aryl Halides
1. Aryl halides are colourless liquids or colourless
solids with characteristic odour.
2. Boiling point generally
increases with increase in the size of aryl group or halogen atom. Boiling
point order
Ar – I > Ar – Br > Ar – Cl > Ar – F
3. The melting point of p-isomer is more than o- and m-isomer. This is because of more symmetrical nature of p-isomer.
4. Due to resonance in
chlorobenzene, C-CI bond is shorter and hence, its dipole moment is less than
that of cyclohexylchloride.
Some Chemical Reactions of Haloarenes
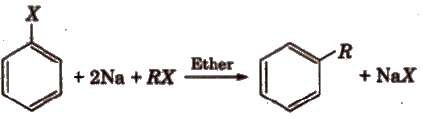
(i) Wurtz
-Fittig reaction:
Here
Halogen atoms from aryl and alkyl part are taken up by Na atoms and further via
free radical mechanism both are joined to each other.
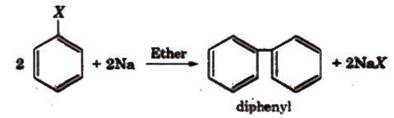
(ii) Fittig
reaction:
Exactly
same as Wurtz Fittig
reactions but No Alkyl Group is taken.