Subsitution Reactions in Haloarenes
1. Nucleophilic Substitution Reaction
Aryl
halides are less reactive towards nucleophilic substitution reaction. Their low
reactivity is attributed due to the following reasons:
1. Due to resonance, C-X bond has partial double
bond character.
2. Stabilisation of
the molecule by delocalisation of electrons.
3. (Instability of phenyl carbocation.
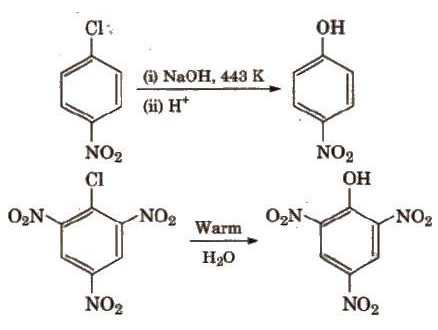
However, aryl halides having
electron withdrawing groups (like – NO2, -SO3H, etc.) at ortho
and para positions undergo nucleophilic substitution reaction easily.

Presence of electron
withdrawing group (-NO2) increases the reactivity.
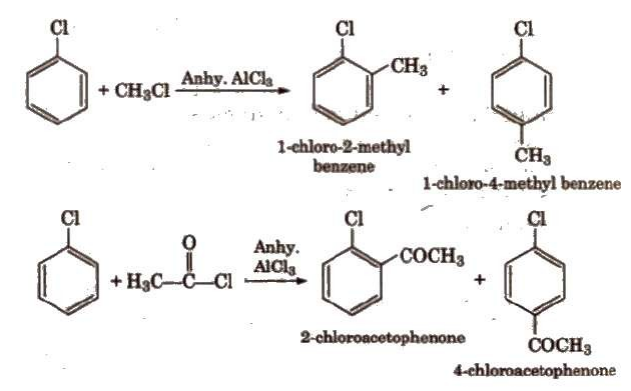
2. Electrophilic Substitution Reactions
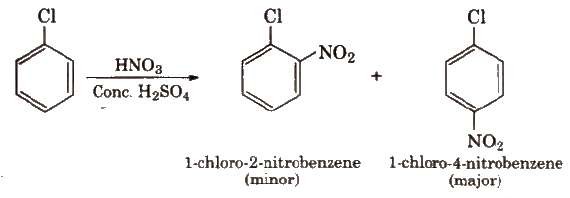
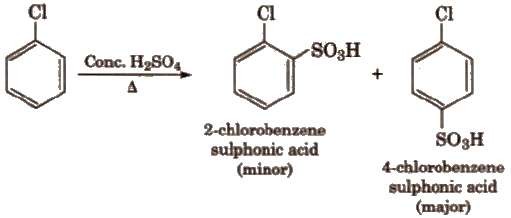
Halogens
are deactivating but o, p-directing. Thus, chlorination, nitration, sulphonation and
Friedel
Craft’s reaction give a mixture of o- and p- chloro
substituted derivatives.
In all
this reactions Electrophile is generated which attacks on benzene ring due its
negative charge holding nature. This particular ion complex is stabilized and
then Electrophile is added as per the Ortho-Para / Meta directing nature of
compound. Mostly para compounds are stable.
For Halogenation X+ is Electrophile [X = Cl, Br, F, I]
Nitration NO2+ is
Electrophile
Sulphonation SO3 is
Electrophile
Fridel Crafts Alkyl / Acyl
group becomes electrophile
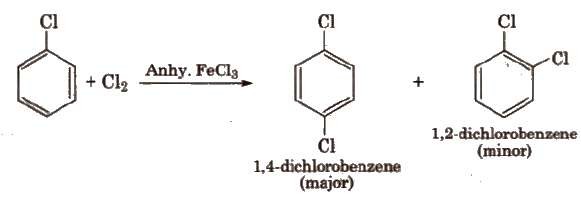
(i) Halogenation
(ii) Nitration
(iii) Sulphonation
(iv) Friedel-Crafts reaction