Proteins
Proteins are the most abundant biomolecules of
the living system. Chief sources of proteins are milk, cheese, pulses, peanuts,
fish, meat, etc. They occur in every part of the body and form the fundamental
basis of structure and functions of life. They are also required for growth and
maintenance of body. The word protein is derived from Greek word, “proteios” which means primary or of prime importance. All
proteins are polymers of α-amino acids.
Amino Acids
There are some 20 amino
acids in the proteins that we consume. These amino acids bond together to form
a larger protein molecule. Amino acid being organic
compound molecules can form various different links with each other
due to the versatile nature of carbon. This enables the great diversity of
proteins that can be found in nature. These are an essential nutrient in our
diet because of the functions they perform.
Structure of Amino Acids
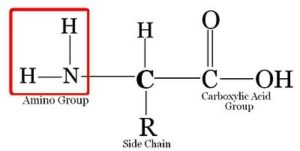
There are actually
thousands of amino acids occurring in nature. But only about 20 amino acids
form a part of the proteins in the human body. These twenty acids will be our
focus here. Although all these have varied structures, the basic structure of
amino acid remains uniform.
Ø All
amino acids contain a carbon atom in the middle of the molecule, the
alpha-carbon
Ø This
atom is surrounded by three chemical groups.
Ø One
is an amine group -NH2
Ø The
second one is a carboxyl group -OOOH
Ø The
third group is denoted by R. This is the variable radical group and is different
for every amino acid. This R group makes the amino acid unique.
Classification
of Amino Acids
Amino Acid can be
classified based on their structure and the structure
of their side chains i.e. the R chains. Now two basic subcategories are
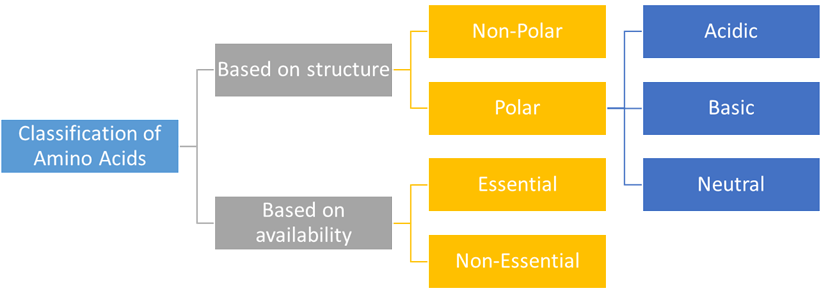
1. Non-Polar
Amino Acids
These are also known
as Hydrophobic. The R group can be either of Alkyl groups (with an alkyl
chain) or Aromatic groups. The acids falling in this group are stated below.
Numbers one to seven are Alkyl and the last two are aromatic
i.
Glycine (H)
ii.
Alanine (CH3)
iii.
Valine (CH(CH3)2)
iv.
Methionine (CH2CH2SCH3)
v.
Leucine (CH2CH(CH3)2)
vi.
Isoleucine (-CH(CH3)CH2CH3)
vii.
Proline
(special structure)
viii.
Phenylalanine
ix.
Tryptophan
2. Polar
Amino Acids
If the side chains of amino
acid contain different polar groups like amines, alcohols or acids they are
polar in nature. These are also known as Hydrophilic Acids. These are further
divided into three further categories.
a) Acidic:
If the side chain contains
an extra element of carboxylic acid component these are acid-polar amino acids.
They tend to donate their hydrogen atom. These are:
i.
Aspartic Acid (CH2COOH)
ii.
Glutamic Acid (CH2CH2COOH)
b) Basic:
These have an extra
nitrogen group that tend to attract a hydrogen atom. The three basic polar
amino acids are
i.
Histidine
ii.
Lysine (CH2(CH2)2NH2)
iii.
Arginine
c) Neutral:
These are neither acidic
nor basic. They have an equal number of amino and carboxyl groups. Also, they
have at least one hydrogen component connected to electronegative atoms. Some
of these neutral acids are
i.
Serine (CH2OH )
ii.
Threonine (CH(OH)CH3)
iii.
Asparagine (CH2OHNH2)
iv.
Glutamine (CH2CH2CONH2)
v.
Cysteine (CH2SH)
vi.
Tyrosine
Amino acid can also be classified on the
basis of their need to the human body and their availability in the
human body
1. Essential
Amino Acids
These are the acids that
cannot be synthesized in our bodies. We must rely on food sources to obtain
these amino acids. They are
Ø Leucine
Ø Isoleucine
Ø Lysine
Ø Theorine
Ø Methionine
Ø Phenylalanine
Ø Valine
Ø Tryptophan
Ø Histidine
(conditionally essential)
2. Non-Essential
These acids are synthesized
in our bodies itself and we need not rely on outside sources for them. They are
either produced in our bodies or obtained from protein breakdowns.
Properties of Amino Acids
Ø Each
amino acid has both an acidic and basic group as you can see from its
structure. This is the reason they behave like salts.
Ø Any
amino acid in the dry state is in crystalline form. They exist as a dipolar
ion. The COOH group exists as an anion. And the NH2 group exists as
a cation. This dipolar ion has a special name “Zwitter
ions’.
Ø In
aqueous solution, alpha amino acids exist in equilibrium between a cationic
form, an anionic form and dipolar ion.
Ø The
Isoelectric point is the pH point at which the concentration of zwitter ions is the highest ad the concentration of
cationic and anionic form is equal. This point is definite for
every α-amino acid.
Ø They
are generally water soluble and also have high melting points.
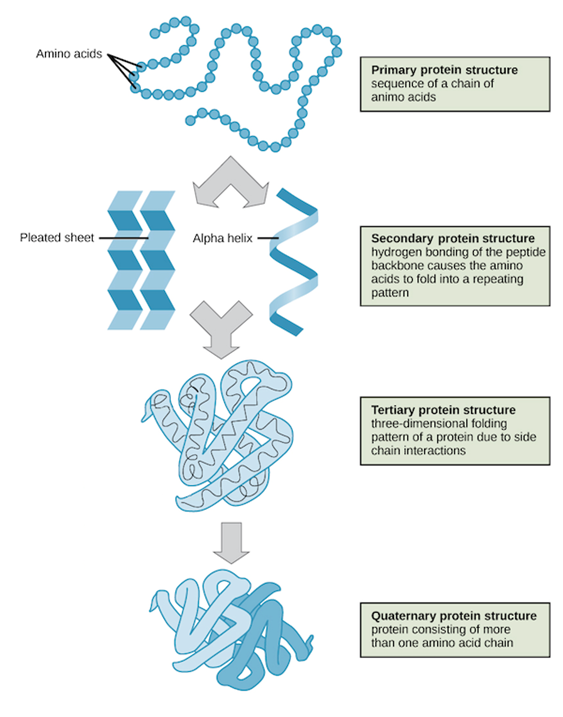
Structure of Proteins
Proteins are what we call biological
polymers (i.e. they occur naturally in nature). Now we previously learnt that
amino acids are the building blocks of proteins. What this actually entails is
that proteins are long chain-like structure, with amino acids being the main
ingredient. These amino acids are connected together with peptide bonds,
and a few such bonds linking together form a polypeptide chain. Now one or more
of these polypeptide chains twist or fold spontaneously and a protein is
formed.
The size of the proteins varies
greatly. It actually depends on the number of polypeptide molecules it
contains. One of the smallest protein molecules is insulin, and the largest
being Titin which consist of 34,350 amino
acids. The four types of protein structure that make up a protein
molecule are:
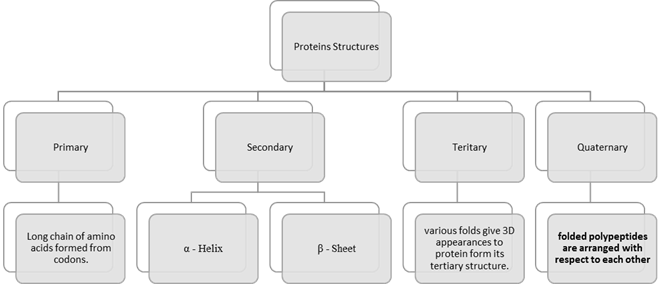
1. Primary
Protein Structure
The primary structure is
the unique formation and order in which the amino acids (the building blocks)
combine and link to give us a protein molecule. Protein gets all its properties
from its primary structure.
There are in all twenty
amino acids in the human body. All of these have a carboxyl group and an amino
group. But each has a different variable group known as the “R” group. It is
this R group that lends a particular protein its unique structure.
Every protein is determined
by the sequencing of the amino acids. The formation and ordering of these amino
acids in proteins are extremely specific. If we alter even one amino acid in
the chain it results in a non-functioning protein or what we call a gene mutation.
2. Secondary
Protein Structure
After the sequencing of
amino acids, we now move on to the secondary structure. This is when the
peptide backbone of the protein structure will fold onto itself, to give
proteins their unique shape. This folding of the polypeptide chains happens due
to the interaction between the carboxyl groups along with the amine groups of
the peptide chains.
There are two kinds of shapes formed in the
secondary structure. These are
·
α-helix: The
backbone follows a helical structure. The hydrogen bonds with the oxygen
between the different layers of the helix, giving it this helical structure.
·
β-pleated
sheet: here the polypeptide chains are stacked next to each other and their
outer hydrogen molecules form intramolecular bonds to give it this sheet-like
structure
3. Tertiary
Structures
This is the structure that
gives protein the 3-D shape and formation. After the amino acids form bonds
(secondary structure) and shapes like helices and sheets, the structure can
coil or fold at random. This is what we call the tertiary structure of
proteins. If this structure is disrupted or disturbed a protein is said to be
denatured which means it is chemically affected and its structure is distorted.
4. Quaternary
Structure
Finally, we come to the fourth
structure. The spatial arrangement of two or more peptide chains leads to this
structure. It is important to note it is not necessary for proteins to have
quaternary structures. Primary, secondary and tertiary structures are present
in all natural proteins, but the same is not true for quaternary structure.
Hence if a protein has only the first three structures it is considered to be a
protein.
Denaturation of
Proteins
Ø Protein found in a biological system
with a unique three-dimensional structure and biological activity is called a
native protein.
Ø When a protein in its native form,
is subjected to physical change like change in temperature or chemical change
like change in pH, the hydrogen bonds are disturbed.
Ø Due to this, globules unfold and helix
get uncoiled and protein loses its biological activity. This is called
denaturation of protein.
Ø During denaturation 2° and 3°
structures are destroyed but 1° structure remains intact.
Ø The coagulation of egg white on
boiling is a common example of denaturation.