Classification
of Polymers
There are several ways of classification of polymers
based on some special considerations. The following are some of the common
classifications of polymers:
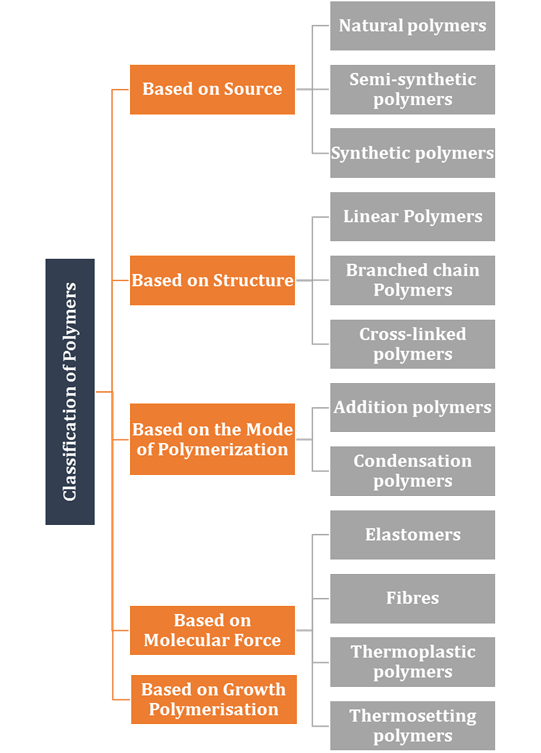
Classification based on source
Natural polymers
Ø
Natural polymers are polymers which occur in
nature
Ø
These polymers are found in plants and animals.
Ø
Examples are proteins, cellulose, starch, some resins
and rubber.
Semi-synthetic
polymers
Ø
Semi-Synthetic polymers are polymers obtained by
making modification in natural polymers artificially in a lab.
Ø
These polymers formed by chemical reaction (in a
controlled environment) and are of commercial importance.
Ø
Cellulose derivatives as cellulose acetate (rayon) and
cellulose nitrate, etc. are the usual examples of this sub category.
Synthetic
polymers
Ø Synthetic polymers are polymers which humans can
artificially create/synthesize in a lab.
Ø These are commercially produced by industries for
human necessities.
Ø Examples of manmade polymers extensively used in daily
life as well as in industry are plastic (polythene), synthetic fibres (Nylon-
6, 6) and synthetic rubbers (Buna - S).
Classification
based on structure of polymers
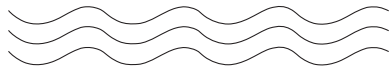
Linear
Polymers
Ø
These polymers consist of long and straight chains.
Ø
The examples are high density polythene, polyvinyl
chloride, etc.
Ø
These chains do not have any side chain.
Ø
These are represented as:
Branched
chain Polymers
Ø
These polymers contain linear chains having some
branches.
Ø
For example, low density polythene.
Ø
These are depicted as follows:
![]()
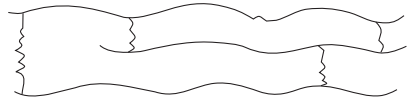
Cross-linked
or Network polymers
Ø
These are usually formed from bi-functional and
tri-functional monomers.
Ø
They contain strong covalent bonds between various
linear polymer chains, e.g. Bakelite, melamine, etc.
Ø
These polymers are depicted as follows:
Addition polymers
Ø
The
addition polymers are formed by the repeated addition of monomer molecules
possessing double or triple bonds, e.g., the formation of polythene from ethene and polypropene from
propene. However, the addition polymers formed by the polymerisation of a
single monomeric species are known as homopolymers,
e.g., polythene.
![]()
Ø
The
polymers made by addition polymerisation from two different monomers are termed
as copolymers, e.g., Buna-S, Buna-N, etc.
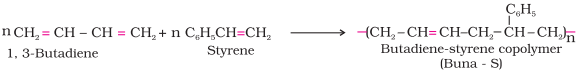
Condensation polymers
Ø The condensation polymers are formed by repeated
condensation reaction between two different bi-functional or tri-functional
monomeric units.
Ø In these polymerisation reactions, the elimination of
small molecules such as water, alcohol, hydrogen chloride, etc. take place.
Ø The examples are terylene
(dacron), nylon 6, 6, nylon 6, etc. For example,
nylon 6, 6 is formed by the condensation of hexamethylene
diamine with adipic acid.
![]()
Classification based
on molecular forces
Elastomers
Ø These are rubber – like solids with elastic
properties.
Ø In these elastomeric polymers, the polymer chains are
held together by the weakest intermolecular forces.
Ø These weak binding forces permit the polymer to be
stretched.
Ø A few ‘crosslinks’ are introduced in between the
chains, which help the polymer to retract to its original position after the
force is released as in vulcanised rubber.
Ø The examples are buna-S, buna-N, neoprene, etc.
Fibres
Ø Fibres are the thread forming solids which possess high
tensile strength and high modulus.
Ø These characteristics can be attributed to the strong
intermolecular forces like hydrogen bonding.
Ø These strong forces also lead to close packing of
chains and thus impart crystalline nature.
Ø The examples are polyamides (nylon 6, 6), polyesters (terylene), etc.
Thermoplastic polymers
Ø These are the linear or slightly branched long chain
molecules capable of repeatedly softening on heating and hardening on cooling.
Ø These polymers possess intermolecular forces of
attraction intermediate between elastomers and fibres.
Ø Some common thermoplastics are polythene, polystyrene,
polyvinyls, etc.
Thermosetting polymers
Ø These polymers are cross linked or heavily branched
molecules, which on heating undergo extensive cross linking in moulds and again
become infusible.
Ø These cannot be reused.
Ø Some common examples are bakelite,
urea-formaldelyde resins, etc.
Classification based on growth
polymerisation
The addition and condensation polymers are nowadays also
referred as chain growth polymers and step growth polymers depending on the
type of polymerisation mechanism they undergo during their formation.
|
Po Polymer |
La large
molecules having high molecular mass formed by combination of number of small
units called monomers. |
|
P Polymerisation |
Th The process of formation of polymers from respective monomers. |
|
Na Natural polymers |
Fofound in plants and animals. Examples: proteins, cellulose, starch. |
|
Sy Synthetic polymers: |
Sy synthesized in laboratory from natural material. Example, nylon 6, 6 , Buna-S |
|
Addition Polymers |
Formed by repeated addition of monomers
having multiple bonds . |
|
H Homopolymers. |
Aa Addition polymers formed from single monomeric species |
|
Copolymers |
A Addition polymers
formed from two different monomeric species |
|
C Condensation polymers |
F Formed by repeated
condensation of different bi or
tri-functional monomer units. |
|
F Fibers |
L Long thin, threadlike
bits of material that are characterized by great tensile (pulling) strength
in the direction of the fibre. The natural fibres – cotton, wool, silk
– are typical. The lining-up is
brought about by drawing – stretching — the return to random looping and
coiling is overcome by strong intermolecular attractions. |
|
E Elastomers |
P Possesses the high
degree of elasticity that is characteristic of rubber: it can be greatly
deformed — stretched to eight times its original length e.g., Buna N and Buna
S, When the stretching
force is removed, the molecular chains of an elastomer do not remain extended
and aligned but return to their original random conformations |
|
T Thermoplastic polymers |
S Soften on heating and
stiffen on Cooling. e.g.
polythene, polystyrene, PVC |
|
Thermosetting polymers |
Do not soften on
heating and cannot be remoulded. Example, Bakelite |