Methods of Polymerisation
There are two broad types of polymerisation reactions,
i.e., the addition or chain growth polymerisation and condensation or step
growth polymerisation.
Addition
Polymerization
Ø In this type of polymerisation,
the molecules of the same monomer or different monomers add together on a large
scale to form a polymer.
Ø The monomers used are
unsaturated compounds, e.g., alkenes, alkadienes
and their derivatives.
Ø This mode of
polymerisation leading to an increase in chain length or chain growth can take
place through the formation of either free radicals or ionic species.
Ø However, the free
radical governed addition or chain growth polymerisation is the most common
mode.
Free
Radical Mechanism
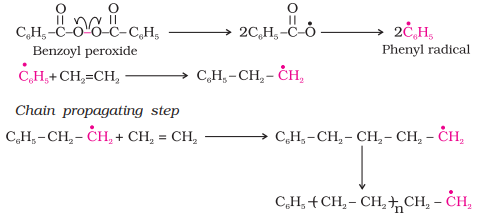
Ø A variety of alkenes or dienes and their derivatives are polymerised in the
presence of a free radical generating initiator (catalyst) like benzoyl
peroxide, acetyl peroxide, tert-butyl peroxide, etc.
Ø The process starts with
the addition of phenyl free radical formed by the peroxide to the ethene double bond thus generating a new and larger free
radical.
Ø This step is called
chain initiating step.
Ø As this radical reacts
with another molecule of ethene, another bigger sized
radical is formed.
Ø The repetition of this
sequence with new and bigger radicals carries the reaction forward and the step
is termed as chain propagating step.
Ø Ultimately, at some
stage the product radical thus formed reacts with another radical to form the
polymerised product.
Ø This step is called the
chain terminating step.
The sequence of steps may be
depicted as follows:
Chain
initiation steps
Chain
terminating step
For termination of the long chain, these free radicals
can combine in different ways to form polythene. One mode of termination of chain
is shown as under:
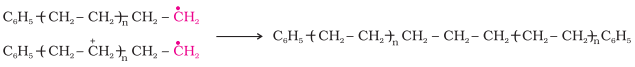
Condensation
Polymerization
Ø This type of
polymerisation generally involves a repetitive condensation reaction between
two bi-functional monomers.
Ø These polycondensation reactions may result in the loss of some simple
molecules as water, alcohol, etc., and lead to the formation of high molecular
mass condensation polymers.
Ø In these reactions, the
product of each step is again a bi-functional species and the sequence of
condensation goes on.
Ø Since, each step produces
a distinct functionalised species and is independent of each other; this
process is also called as step growth polymerisation.
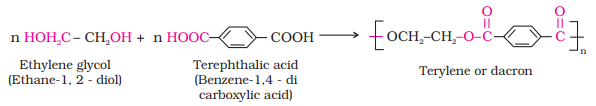
Ø The formation of terylene or dacron
by the interaction of ethylene glycol and terephthalic
acid is an example of this type of polymerisation.
Copolymerisation
Ø Copolymerisation is a
polymerisation reaction in which a mixture of more than one monomeric species
is allowed to polymerise and form a copolymer.
Ø The copolymer can be
made not only by chain growth polymerisation but by step growth polymerisation
also.
Ø It contains multiple
units of each monomer used in the same polymeric chain.
Ø For example, a mixture
of 1, 3 – butadiene and styrene can form a copolymer.
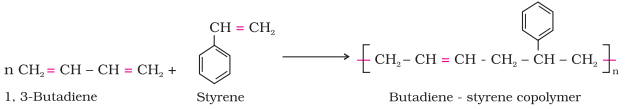
Ø
Copolymers have properties quite different from homopolymers.
Ø
For example, butadiene - styrene copolymer is quite
tough and is a good substitute for natural rubber.
Ø
It is used for the manufacture of autotyres,
floortiles, footwear components, cable insulation,
etc.