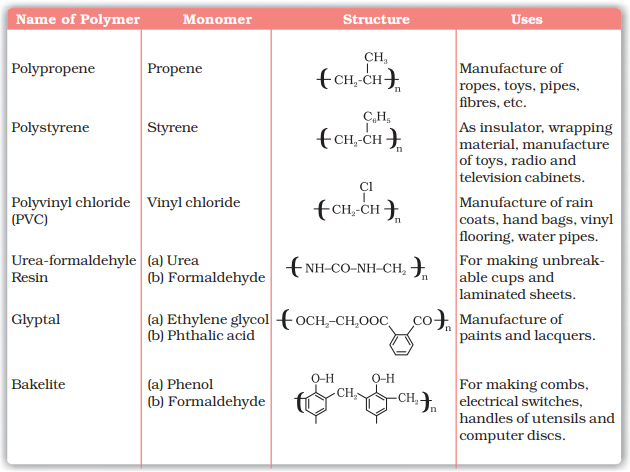
Some Important Polymers
Non – Biodegradable Polymers
1. Polythene
There
are two types of polythene as given below:
i.
Low density polythene:
·
It is obtained by the polymerisation of ethene under high pressure of 1000 to 2000 atmospheres at a
temperature of 350 K to 570 K in the presence of traces of dioxygen
or a peroxide initiator (catalyst).
·
The low density polythene (LDP) obtained through the
free radical addition and H-atom abstraction has highly branched structure.
·
Low density polythene is chemically inert and tough
but flexible and a poor conductor of electricity.
·
Hence, it is used in the insulation of electricity
carrying wires and manufacture of squeeze bottles, toys and flexible pipes.
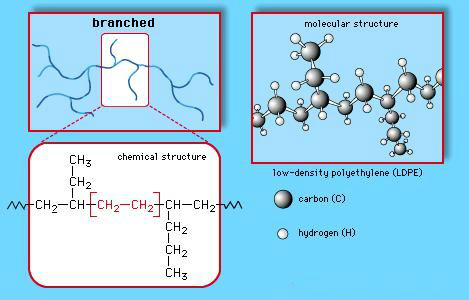
Low
Density Polyethene (LDPE) structures
ii.
High density polythene:
·
It is formed when addition polymerisation of ethene takes place in a hydrocarbon solvent in the presence
of a catalyst such as triethylaluminium and titanium
tetrachloride (Ziegler-Natta catalyst) at a temperature of 333 K to 343 K and
under a pressure of 6-7 atmospheres.
·
High density polythene (HDP) thus produced, consists
of linear molecules and has a high density due to close packing.
·
It is also chemically inert and more tough and hard.
It is used for manufacturing buckets, dustbins, bottles, pipes, etc.
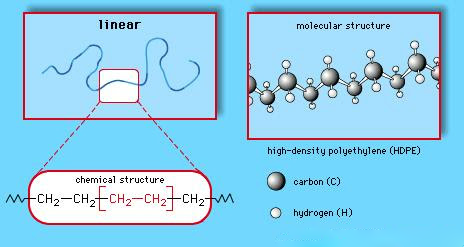
High
Density Polyethene (HDPE) structures
2. Polytetrafluoroethene (Teflon)
·
Teflon is manufactured by heating tetrafluoroethene
with a free radical or persulphate catalyst at high
pressures.
·
It is chemically inert and resistant to attack by
corrosive reagents.
·
It is used in making oil seals and gaskets and also
used for non – stick surface coated utensils.

3. Polyacrylonitrile
·
The addition polymerisation of acrylonitrile in
presence of a peroxide catalyst leads to the formation of polyacrylonitrile.
·
Polyacrylonitrile is used as a substitute
for wool in making commercial fibres as orlon
or acrilan.
·
Acrylic fibres have good resistance to stains,
chemicals, insects and fungi.

4. Polyamides
·
These polymers possessing amide linkages are important
examples of synthetic fibres and are termed as nylons.
·
The general method of preparation consists of the
condensation polymerisation of diamines with dicarboxylic acids and also of amino acids and their
lactams.
Preparation of nylons
i.
Nylon 16,6:
·
It is prepared by the condensation polymerisation of hexamethylenediamine with adipic
acid under high pressure and at high temperature.
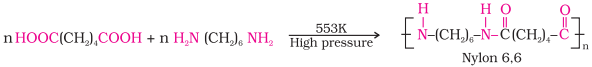
·
Nylon 6, 6 is used in making sheets, bristles for
brushes and in textile industry.
ii.
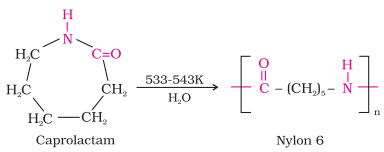
Nylon 6:
·
It is obtained by heating caprolactum
with water at a high temperature.
·
Nylon 6 is used for the manufacture of tyre cords,
fabrics and ropes.
5. Polyesters
·
These are the polycondensation
products of dicarboxylic acids and diols.
·
Dacron or terylene
is the best known example of polyesters.
·
It is manufactured by heating a mixture of ethylene
glycol and terephthalic acid at 420 to 460 K in the
presence of zinc acetateantimony trioxide catalyst as
per the reaction given earlier.
·
Dacron fibre (terylene)
is crease resistant and is used in blending with cotton and wool fibres and
also as glass reinforcing materials in safety helmets, etc.
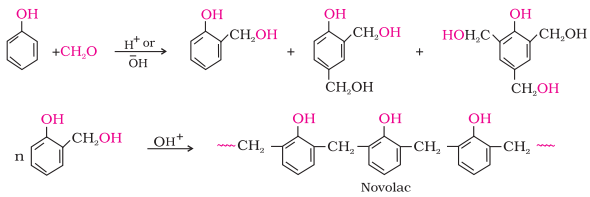
6. Phenol - formaldehyde polymer
(Bakelite and related polymers)
·
Phenol - formaldehyde polymers are the oldest
synthetic polymers.
·
These are obtained by the condensation reaction of
phenol with formaldehyde in the presence of either an acid or a base catalyst.
·
The reaction starts with the initial formation of o-and/or
p-hydroxymethylphenol derivatives, which
further react with phenol to form compounds having rings joined to each other
through –CH2 groups.
·
The initial product could be a linear product – Novolac used in paints.
·
Novolac on heating with
formaldehyde undergoes cross linking to form infusible solid mass called bakelite.
·
It is used for making combs, phonograph records,
electrical switches and handles of various utensils.
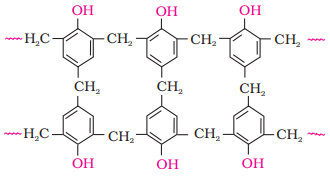
Bakelite
structure
7. Rubber:
Natural rubber:
·
Rubber
is a natural polymer and possesses elastic properties. It is also termed as
elastomer and has a variety of uses.
·
It is
manufactured from rubber latex which is a colloidal dispersion of rubber in
water.
·
This
latex is obtained from the bark of rubber tree and is found in India, Srilanka, Indonesia, Malaysia and South America.
·
Natural
rubber may be considered as a linear polymer of isoprene (2-methyl-1,
3-butadiene) and is also called as cis - 1, 4 - polyisoprene.
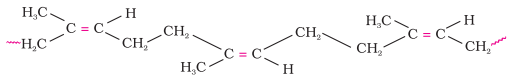
·
The cis-polyisoprene
molecule consists of various chains held together by weak van der Waals
interactions and has a coiled structure. Thus, it can be stretched like a
spring and exhibits elastic properties.
Vulcanisation of rubber:
·
Natural
rubber becomes soft at high temperature (>335 K) and brittle at low
temperatures (<283 K) and shows high water absorption capacity.
·
It is
soluble in non-polar solvents and is non-resistant to attack by oxidising
agents.
·
To
improve upon these physical properties, a process of vulcanisation is carried
out.
·
This
process consists of heating a mixture of raw rubber with sulphur and an
appropriate additive at a temperature range between 373 K to
415 K.
·
On
vulcanisation, sulphur forms cross links at the reactive sites of double bonds
and thus the rubber gets stiffened.
·
In the
manufacture of tyre rubber, 5% of sulphur is used as acrosslinking
agent.
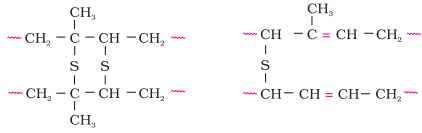
Synthetic rubbers
·
Synthetic
rubber is any vulcanisable rubber like polymer, which
is capable of getting stretched to twice its length.
·
However,
it returns to its original shape and size as soon as the external stretching
force is released.
·
Thus,
synthetic rubbers are either homopolymers of 1, 3 -
butadiene derivatives or copolymers of 1, 3 - butadiene or its derivatives with
another unsaturated monomer.
Preparation of Synthetic Rubbers
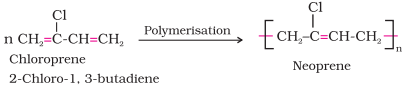
1.
Neoprene
·
Neoprene
or polychloroprene is formed by the free radical
polymerisation of chloroprene.
·
It has
superior resistance to vegetable and mineral oils. It is used for manufacturing
conveyor belts, gaskets and hoses.
2. Buna – N
·
Buna –N
is obtained by the copolymerisation of 1, 3 – butadiene and acrylonitrile in
the presence of a peroxide catalyst.
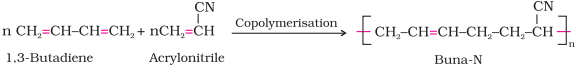
·
It is resistant to the action of petrol, lubricating
oil and organic solvents. It is used in making oil seals, tank lining, etc.
Biodegradable Polymers
·
A large number of polymers are quite resistant to the
environmental degradation processes and are thus responsible for the
accumulation of polymeric solid waste materials.
·
These solid wastes cause acute environmental problems
and remain undegraded for quite a long time.
·
In view of the general awareness and concern for the
problems created by the polymeric solid wastes, certain new biodegradable
synthetic polymers have been designed and developed.
·
These polymers contain functional groups similar to
the functional groups present in biopolymers.
·
Aliphatic polyesters are one of the important classes
of biodegradable polymers.
Some important examples are:
Poly ꞵ-hydroxybutyrate
– co-ꞵ-hydroxy
valerate (PHBV)
·
It is obtained by the copolymerisation of
3-hydroxybutanoic acid and 3 - hydroxypentanoic acid.
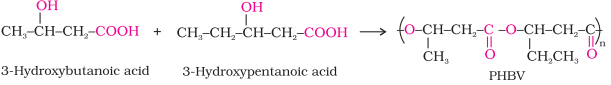
·
PHBV is used in speciality packaging, orthopaedic
devices and in controlled release of drugs.
·
PHBV undergoes bacterial degradation in the
environment.
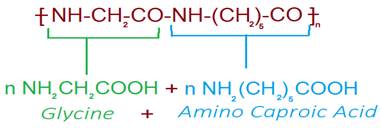
Nylon 2–nylon 6
·
It is an alternating polyamide copolymer of glycine
(H2N–CH2–COOH) and amino caproic acid [H2N(CH2)5COOH]
·
It is biodegradable.
Some Other Commercially
Important Polymers