Conductance of Electrolytic Solutions
Resistance of a cell
The
resistance R of a cell depends
i.
Directly
on the length of column (distance between the electrodes) of solution, l i.e.
R ∝ l
ii.
Inversely
on area of cross-section A of the electrode plates, i.e.
R ∝ ![]()
iii.
On the
nature of the electrolyte
On
combining the above factors, we get
R = ρ × ![]()
The
proportionality constant ρ (Rho) is called resistivity of electrolyte.
R can
be calculated using wheat stone bridge.
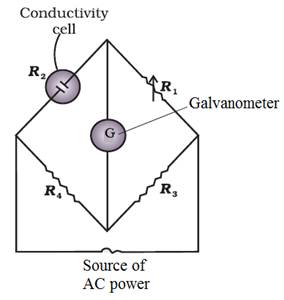
When the
Wheatstone bridge is balanced
R2 = ![]()
Conductance of a cell
It is the ease of flow of electric current
through the conductor. It is reciprocal of resistance (R).
G = ![]()
= ![]()
= κ × ![]()
where,
κ = ![]() , is called the conductivity
, is called the conductivity
The SI unit of conductance is siemens,
represented by the symbol ‘S’ and is equal to ohm–1 (also known
as mho) or Ω–1.
Conductivity of a cell (κ)
The inverse of resistivity is called conductivity or specific
conductance. It is represented by the symbol, κ (Greek, kappa).
κ = ![]()
= ![]()
= G × ![]()
κ = G × G*
= ![]()
where,
G* = ![]() is called the cell constant
is called the cell constant
The SI unit is S m-1
Unit of cell constant is cm-1 or m-1.
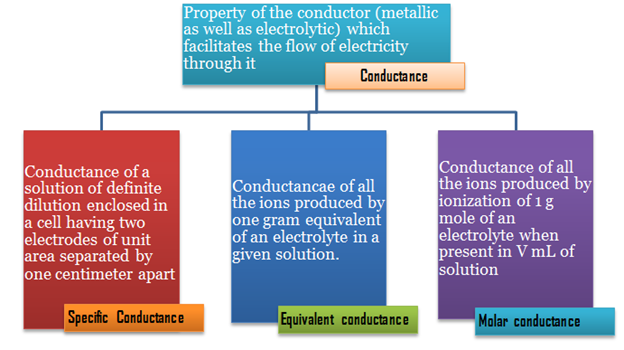
Molar
conductivity of a solution (Λm)
Molar conductivity of a solution at a given concentration is defined as,
Λm = ![]()
= ![]()
where
M = molarity
c = concentration in mole
per m3
The unit of molar conductivity is Ω-1 cm2 mol-1 or
S cm2 mol-1.
1 S m2 mol–1 =
104 S cm2 mol–1
or 1 S cm2 mol–1 =
10–4 S m2 mol–1.
Factors
affecting conductivity
i.
Nature of electrolyte - The strong electrolytes like KNO3,
KCl, NaOH etc. are
completely ionised in aqueous solution and have high values of molar
conductivity.
The weak electrolytes are ionised to a lesser
extent in aqueous solution and have lower values of molar conductivity.
ii.
Concentration of the solution - The concentrated solutions
of strong electrolytes have significant interionic attractions, which reduce
the speed of ions and lower the value of Λm.
As the dilution decreases such attractions
also decrease which increases the value of Λm.
iii.
Temperature - The increase of temperature decreases inter-ionic attractions
and increases kinetic energy of ions and their speed. Thus, Λm and
Λeq increase with temperature.
iv.
Size of the ions produced and their solvation.
v.
The nature of the solvent and its viscosity. Higher the
viscosity, lower is conductivity
Variation of
conductivity and molar conductivity with concentration
Conductivity
of strong electrolytes
For strong electrolytes, Λm increases slowly with
dilution and is given by,
Λm= ![]() − A√C
− A√C
If we plot Λm against √C,
we obtain a straight line with intercept equal to ![]() and slope equal to ‘–A’.
The value of ‘A’ for a given solvent and temperature depends on the type of
electrolyte i.e., the charges on the cation and anion
produced on the dissociation of the electrolyte in the solution.
and slope equal to ‘–A’.
The value of ‘A’ for a given solvent and temperature depends on the type of
electrolyte i.e., the charges on the cation and anion
produced on the dissociation of the electrolyte in the solution.
The limiting value, ![]() (the molar
conductivity at zero concentration or at infinite dilution) can be obtained
extrapolating the graph.
(the molar
conductivity at zero concentration or at infinite dilution) can be obtained
extrapolating the graph.
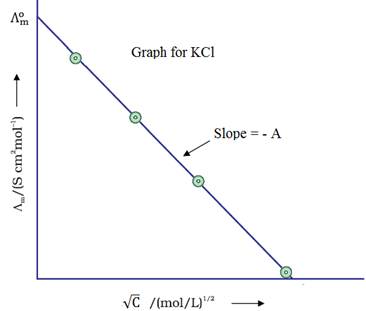
Depending upon the type of ions involved, NaCl,
CaCl2, MgSO4 are known as 1-1, 2-1 and 2-2
electrolytes respectively.
All electrolytes of a particular type have the same value for ‘A’.
Conductivity
of weak electrolytes
For weak electrolytes like acetic acid, Λm increases steeply on dilution,
especially near lower concentrations. This is because they have lower degree of
dissociation at higher concentrations and hence for such electrolytes, the
change in Λm with dilution is
due to increase in the degree of dissociation and consequently the number of
ions in total volume of solution that contains 1 mol
of electrolyte.
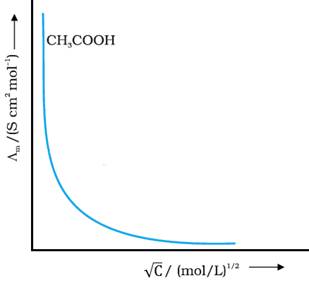
Therefore, ![]() cannot be obtained by
extrapolation of
cannot be obtained by
extrapolation of ![]() to zero
concentration.
to zero
concentration.
At infinite dilution (i.e., concentration c → zero) electrolyte
dissociates completely (α =
1), but at such low concentration the conductivity of the solution is so low
that it cannot be measured accurately.
Therefore, ![]() for weak electrolytes
is obtained by using Kohlrausch’s law
of independent migration of ions.
for weak electrolytes
is obtained by using Kohlrausch’s law
of independent migration of ions.