Electrochemistry
Electrochemistry is the branch of chemistry which deals with the study
of production of electricity from energy released during spontaneous chemical
reactions and the use of electrical energy to bring about non-spontaneous
chemical transformations.
Importance of electrochemistry
1.
Production
of metals like Na, Mg, Ca and Al.
2.
Electroplating.
3.
Purification
of metals.
4.
Batteries
and cells used in various instruments.
Conductors
Substances
that allow electric current to pass through them are known as conductors.
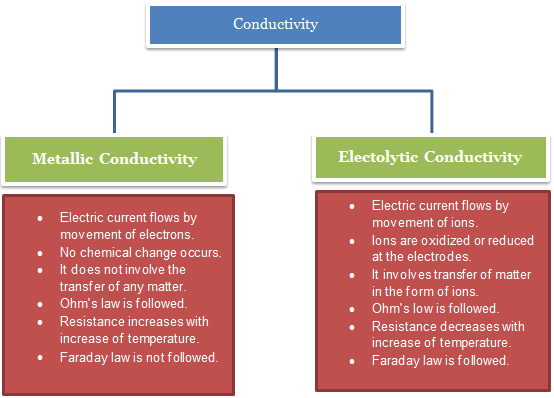
|
Metallic
Conduction |
Electrolytic
Conduction |
|
Electric current flows by
movement of electrons. |
Electric current flows by
movement of ions. |
|
No chemical change occurs. |
Ions are oxidized or
reduced at the electrodes. |
|
It does not involve the
transfer of any matter. |
It involves transfer of
matter in the form of ions. |
|
Ohm's law is followed. |
Ohm's low is followed. |
|
Resistance increases with
increase of temperature. |
Resistance decreases with
increase of temperature. |
|
Faraday law is not
followed. |
Faraday law is followed. |
Metallic conductors or electronic conductors
Substances which allow the electric current to pass through them by the
movement of electrons are called metallic conductors, e.g., metals.
Electrolytic conductors or electrolytes
Substances which allow the passage of electricity through their fused
state or aqueous solution and undergo chemical decomposition, are called
electrolytic conductors, e.g., aqueous solution of acids, bases and salts.
Electrolytes
are of two types -
Strong electrolytes
The electrolytes that completely dissociate or ionise into ions are
called strong electrolytes. e.g., HCl, NaOH, K2SO4.
Weak electrolytes
The electrolytes that dissociate partially (α < 1) are called weak
electrolytes, e.g., CH3COOH, H2CO3, NH4OH,
H2S, etc.