Corrosion
Slow formation of undesirable compounds such as oxides, sulphides or
carbonates at the surface of metals by reaction with moisture and other
atmospheric gases is known as corrosion.
Factors affecting corrosion
i.
Reactivity
of metals.
ii.
Presence
of moisture and atmospheric gases like CO2, SO2, etc.
iii.
Presence
of impurities.
iv.
Presence
of electrolyte.
Electrochemical theory of rusting of iron
An electrochemical cell, also known as corrosion cell, is developed at
the surface of iron.
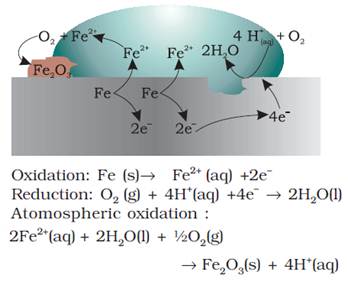
At a particular
spot of an object made of iron, oxidation takes place and that spot behaves as
anode.
Anode - Pure iron
Anode
reaction: 2Fe(s) →
2 Fe2+ + 4e– , ![]() = –
0.44 V
= –
0.44 V
Electrons released at anodic spot move through the metal and go to
another spot on the metal and reduce oxygen in presence of H+.
H+ is believed to be available from H2CO3 formed
due to dissolution of carbon dioxide from air into water. Hydrogen ion in water
may also be available due to dissolution of other acidic oxides from the
atmosphere.
This spot
behaves as cathode.
Cathode - Impure iron surface
Cathode
reaction: O2(g) + 4H+(aq)
+ 4e– → 2H2O(l), ![]() =
1.23V
=
1.23V
The
overall reaction:
2Fe(s) + O2(g) + 4H+(aq)
→ 2Fe2+(aq) + 2H2O(l), Eocell =1.67 V
Prevention of
rusting of iron:
Rusting
of iron can be prevented by the following methods:
1.
Barrier
protection through coating of paints or electroplating.
2.
Through
galvanisation or coating of surface with tin metal.
3.
By the
use of antirust solutions (bis phenol).
4.
By cathodic protection in which a metal is protected from
corrosion by connecting it to another metal that is more easily oxidised.