Fuel Cells
Galvanic cells that are designed to convert the energy
of combustion of fuels like hydrogen, methane, methanol, etc. directly into
electrical energy are called fuel
cells.
Hydrogen
oxygen fuel cell
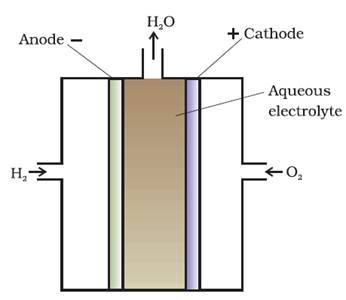
Fuel cell using O2 and
H2
Electrodes - Made of porous graphite impregnated with
catalyst (Pt, Ag or a metal oxide).
Electrolyte - Aqueous solution of KOH or NaOH
Oxygen and hydrogen are continuously fed into the
cell.
Reactions
Cathode: O2(g) + 2H2O(l) + 4e– → 4OH–(aq)
Anode: 2H2(g) + 4OH–(aq) →
4H2O(l)
+ 4e–
Overall
reaction:
2H2(g) + O2(g)
→ 2 H2O(l
)
Efficiency
of cell - 70%, Efficiency
of thermal plants – 40%
Cell
potential - 1.23 V