Electrochemical
Cell
Galvanic cell or Voltaic cell or electrochemical cell converts the chemical
energy of a spontaneous redox reaction into electrical energy.
In the Galvanic cell, the Gibbs energy of
the spontaneous redox reaction is converted into electrical
work which may be used for running motor or other electrical gadgets like
heater, fan, geyser, etc.
Difference between an electrolytic cell and a
galvanic cell
|
Electrochemical cell (Galvanic
Cell) |
Electrolytic cell |
|
A Galvanic cell
converts chemical energy into electrical energy. |
An electrolytic cell converts electrical energy
into chemical energy. |
|
The redox reaction is
spontaneous and is responsible for the production of electrical energy. |
The redox reaction
is not spontaneous and electrical energy has to be supplied to initiate the
reaction. |
|
The two half-cells are
set up in different containers, being connected through the salt bridge or
porous partition. |
Both the electrodes are placed in a same container
in the solution or molten electrolyte. |
|
The anode is negative
and cathode is positive electrode. The reaction at the anode is oxidation and
that at the cathode is reduction. |
The anode is
positive and cathode is the negative electrode. The reaction at the anode is
oxidation and that at the cathode is reduction. |
|
The electrons are
supplied by the species getting oxidized. They move from anode to the cathode
in the external circuit. |
The external battery supplies the electrons. They
enter through the cathode and come out through the anode. |
Daniel cell
An electrochemical cell using zinc and copper metals as electrodes, is
known as Daniell cell.
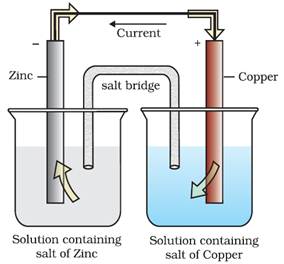
Zn(s) + Cu2+(aq)
→ Zn2+(aq) + Cu(s)
It has an electrical potential equal to 1.1V when concentration of Zn2+ and
Cu2+ ions is unity (1 mol dm–3).
If an external opposite potential is applied in the galvanic cell and
increased slowly, the reaction continues till the opposing voltage reaches the
value 1.1V. The reaction stops altogether when the external opposing voltage
reaches 1.1 Volt and no current flows through the cell.
Any further increase in the external potential again starts the reaction
but in the opposite direction. It now functions as an electrolytic
cell, a device for using electrical energy to carry non-spontaneous chemical
reactions.
When Eext <
1.1 V
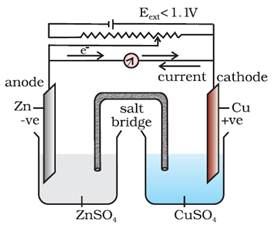
i.
Electrons
flow from Zn rod to Cu rod hence current flows from Cu to Zn.
ii.
Zn
dissolves at anode and copper deposits at cathode.
When Eext =
1.1 V
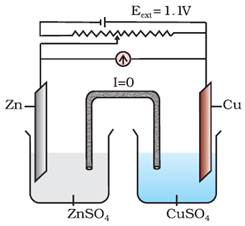
i.
No flow
of electrons or current.
ii.
No
chemical reaction.
When Eext >
1.1 V
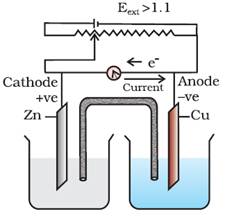
i.
Electrons
flow from Cu to Zn and current flows from Zn to Cu.
ii.
Zinc is
deposited at the zinc electrode and copper dissolves at copper electrode
Cell half reactions
The reaction is a combination of two half reactions whose addition gives
the overall cell reaction:
i.
Cu2+ +
2e– → Cu(s) (reduction half reaction)
ii.
Zn(s) →
Zn2+ + 2e– (oxidation half reaction)
The reduction half reaction occurs on the copper
electrode while the oxidation half reaction occurs on
the zinc electrode.
These two
portions of the cell are called half-cells or redox
couples.
The
copper electrode is called the reduction half-cell and the
zinc electrode, the oxidation half-cell.