Batteries
These are
source of electrical energy which may have one or more cells connected in
series.
For a
good quality battery it should be reasonably light, compact and
its voltage should not vary appreciably during its use.
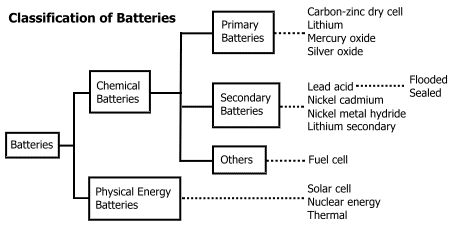
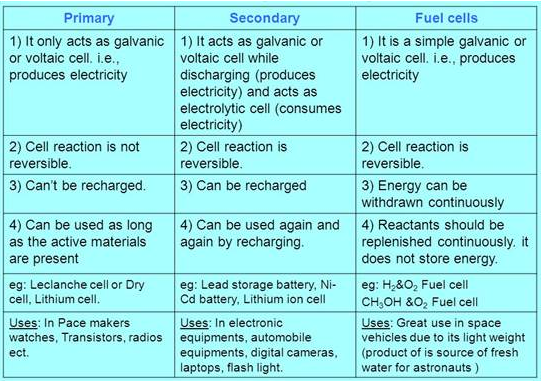
Primary batteries
In primary batteries, the reaction occurs only once and after use over a
period of time battery becomes dead and cannot be reused.
(i) Dry cell or Leclanche cell
The cell consists of a zinc container that also
acts as anode and the cathode is a carbon
(graphite) rod surrounded by powdered manganese dioxide and carbon.
The space between the electrodes is filled by a moist paste of ammonium
chloride (NH4Cl) and zinc chloride (ZnCl2).
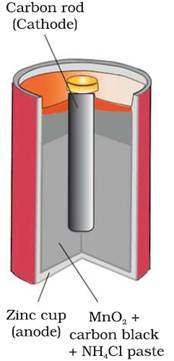
The
electrode reactions can be written approximately as,
Anode: Zn(s) → Zn2+ +
2e –
Cathode: MnO2 + NH4+ +
e– → MnO(OH) + NH3
In the
reaction at cathode, manganese is reduced from the +4 oxidation state to the +3
state.
Ammonia
produced in the reaction forms a complex with Zn2+ to give [Zn(NH3)4]2+.
The cell
has a potential of nearly 1.5V.
(ii) Mercury cell
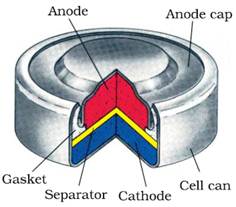
Mercury cell, suitable for low current devices like hearing aids,
watches, etc. consists of zinc – mercury amalgam as anode and
a paste of HgO and carbon as the cathode.
The electrolyte is
a paste of KOH and ZnO.
The electrode
reactions for the cell are,
Anode: Zn(Hg) + 2OH–→
ZnO(s) + H2O + 2e–
Cathode: HgO + H2O
+ 2e– → Hg(l) + 2OH–
The
overall reaction:
Zn(Hg) + HgO(s) → ZnO(s) + Hg(l)
Cell
potential for mercury cell is 1.35 V
Secondary batteries
These cells can be recharged and can be used again and again
(i) Lead storage battery
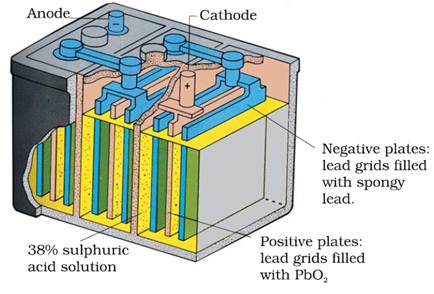
Used in
automobiles and invertors.
Anode-Spongy lead
Cathode-Grid of lead packed with PbO2
Electrolyte - 38% H2SO4 by
mass
The cell
reactions when the battery is in use or discharge
reaction
Anode: Pb(s) + SO42–(aq) → PbSO4(s) + 2e–
Cathode: PbO2(s) + SO42–(aq) + 4H+(aq) + 2e– → PbSO4 (s)
+ 2H2O (l)
Overall
cell reaction consisting of cathode and anode reactions is:
Pb(s) + PbO2(s)
+ 2H2SO4(aq)
→ 2PbSO4(s) + 2H2O(l)
When
recharged the cell reactions are reversed.
(ii) Nickel cadmium storage cell
Has longer life than the lead storage cell
but more expensive to manufacture.
Anode-Cadmium
Cathode-Metal grid containing NiO2
Electrolyte - KOH solution
Reactions
during discharge
Anode: Cd(s) + 2OH-(aq)
→ Cd(OH)2(s) + 2e-
Cathode: NiO2(s) + 2H2O(l)
+ 2e- → Ni(OH)2(s) + 2OH-(aq)
Overall
reaction,
Cd (s) + 2Ni(OH)3 (s) → CdO
(s) + 2Ni(OH)2 (s) + H2O (l )
Cell
potential for Nickel cadmium storage cell is 1.4 V