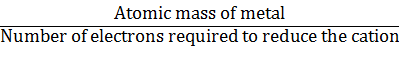Faraday’s Laws of
Electrolysis
Faraday’s
first law of electrolysis
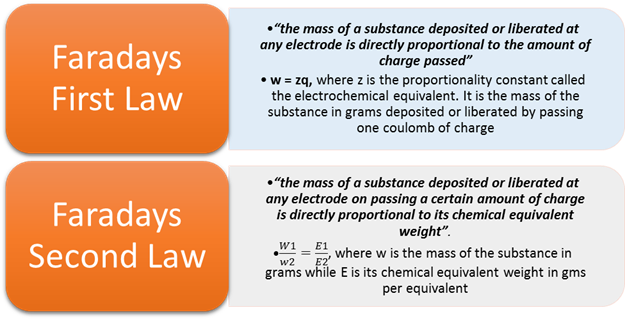
The amount of chemical reaction which occurs at any
electrode during electrolysis by a current is proportional to the quantity of electricity
passed through the electrolyte (solution or melt).
Or
The
amount of the substance deposited or liberated at cathode is directly
proportional to the quantity of electricity passed through electrolyte.
W ∝ I ×
t
or W = I
t Z = Q Z
Here, I is the
current in amp, t
is time in sec, Q is quantity of
charge (coulomb) and Z is a constant
known as electrochemical
equivalent.
When I = 1
amp, t = 1 sec then Q = 1 coulomb, then W = Z.
Thus, electrochemical equivalent is the
amount of the substance deposited or liberated by passing 1A current for 1 sec
(i.e. 1 coulomb, I × t = Q).
Faraday’s
second law of electrolysis
The amounts of different substances liberated by the
same quantity of electricity passing through the electrolytic solution are proportional
to their chemical equivalent weights or
Hence,
electrochemical equivalent ∝ eq. weight
The amount of electricity (or charge) required for oxidation
or reduction depends on the stoichiometry of the electrode reaction.
For example, in the reaction,
Ag+(aq) + e– → Ag(s)
One mole of the electron is required for the reduction of one mole of
silver ions.
Now, the charge on one electron is 1.6021 × 10–19 C.
Therefore, the charge on one mole of electrons
= NA × 1.6021 × 10–19 C
= 6.02 × 1023 mol–1 ×
1.6021×10–19 C
= 96487 C mol–1 ≈ 96500
C mol–1
This quantity of electricity is called Faraday and is
represented by the symbol F.
For the electrode reactions
Mg2+ (l) + 2e– →
Mg(s)
One mole of Mg2+ requires 2 moles of electrons (2F).
And for
Al3+ (l) + 3e– →
Al(s)
One mole of Al3+ requires 3 moles of electrons (3F).
The charge passed through the electrolytic cell during electrolysis is
equal to the product of current in
amperes and time in seconds.
In commercial production of metals, current as high as 50,000 amperes is
used that amounts to about 0.518 F per second.