Chemical
Kinetics
The
branch of chemistry, which deals with the rate of chemical
reactions, the factors affecting the rate of reactions and
the mechanism of the reactions, is called chemical
kinetics.
We need
to find out,
a.
the feasibility
of a chemical reaction which can be predicted by thermodynamics (A reaction
with ΔG < 0, at constant temperature and pressure is feasible);
b.
extent to
which a reaction will proceed can be determined from chemical equilibrium;
c.
speed of a
reaction i.e., time taken by a reaction to reach equilibrium.
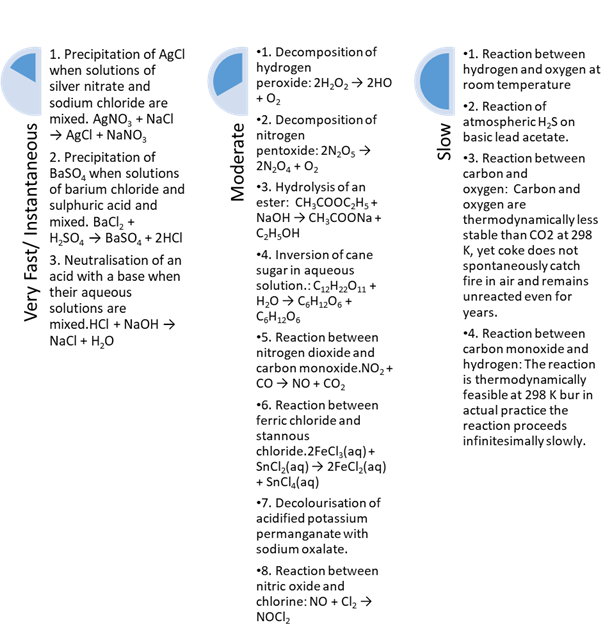
Classification of chemical reactions on the basis
of rate of reaction
Fast/instantaneous reactions
Chemical reaction which completes in less than 1 ps
(10-12 s) time is known as fast reaction. It is practically
impossible to measure the speed of such reactions, e.g., ionic reactions,
organic substitution reactions.
Slow reactions
Chemical reactions which complete in a long time from some minutes to
some years are called slow reactions, e.g., rusting of iron, transformation of
carbon into diamond etc.
Moderately slow reactions
Chemical reactions which are intermediate between slow and fast
reactions are called moderately slow reactions.