Integrated Rate Equation
The
concentration dependence of rate is called differential rate equation. It is not
always convenient to determine the instantaneous rate, as it is measured by
determination of slope of the tangent at point, t in concentration vs time plot. This makes it difficult to determine the rate
law and hence the order of the reaction.
If we integrate the
differential rate equation to give a relation between directly
measured experimental data, i.e., concentrations at different times and rate
constant, the equation obtained is called integrated rate equation.
The
integrated rate equations are different
for the reactions of different reaction orders.
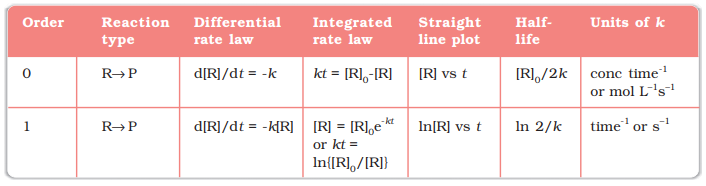
Zero order reactions
The rate of
the reaction is proportional to zero power of the concentration of reactants.
Consider the reaction, R →
P
![]()
![]()
![]()
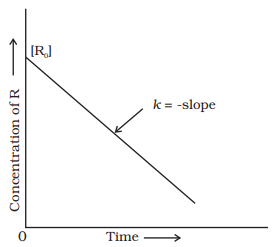
Integrating both sides, we get,
[R] = – k t + C
where, C is the
constant of integration.
Now, at t = 0, the concentration
of the reactant R = [Ro] = initial concentration of the reactant.
Substituting the value, we get,
[Ro] = –k × 0 + C
[Ro] = C
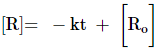
![]()
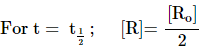
![]()
For zero order gaseous reactions,
![]()
Example
![]()
Rate = k[NH3]0 = k
First
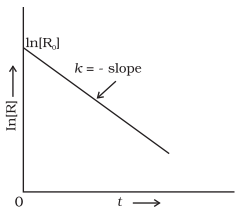
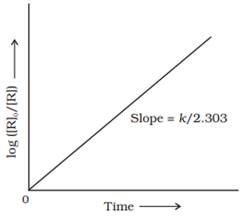
order reactions
The reaction, whose rate is
proportional to the concentration of reactants, is called first order reaction.
R → P
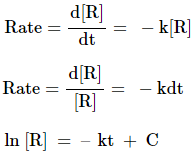
When t = 0, R = [Ro],
where [Ro] is the initial concentration of the reactant.
Therefore,
ln [Ro] = – k × 0 + C
ln [Ro] = C
Substituting the value of C
![]()
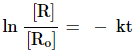
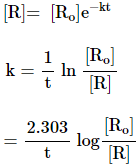
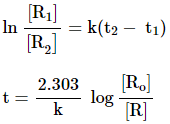
![]()
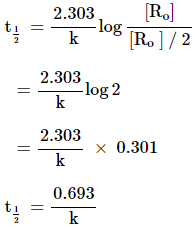
Ø
For zero order reaction, t½ ∝
[R]o.
Ø
For first order reaction t½ is
independent of [R]o.
For first order gas phase reaction
of type
A(g) → B(g) + C(g)
Let pi be the
initial pressure of A and pt the
total pressure at time ‘t’.
Total pressure pt = pA +
pB + pC (pressure units)
If x atm be the decrease in pressure of A at time t and one mole
each of B and C is being formed, the increase in pressure of B and C will also
be x atm each.
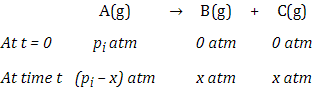
Now
pt = (pi –
x) + x + x
= pi +
x
x = (pt - pi)
where,
pA = pi –
x = pi – (pt – pi)
pA = 2pi –
pt
![]()
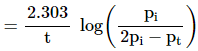
Example:
Hydrogenation of ethene is an example of first order reaction.
C2H4(g) + H2 (g) →
C2H6(g)
Rate = k [C2H4]
All natural and
artificial radioactive decay of unstable nuclei take place by first
order kinetics.
![]()
Rate = k [Ra]
Half life of reaction (t1/2)
The time
in which the concentration of a reactant is reduced to one half of its initial
concentration.
Eg: For a Zero Order
reaction t1/2 = ![]()
Eg: For a first order
reaction t1/2 = ![]() log2 =
log2 = ![]()
Pseudo
first order reaction
Chemical
reactions which appear to be of higher order but actually are of the lower
order are called pseudo order reactions. In case of pseudo
first order reaction, chemical reaction between two substances takes place when
one of the reactants is present in excess, e.g., hydrolysis of ester.
Example – Hydrolysis of ethyl
acetate
During the
hydrolysis of 0.01 mol of ethyl acetate with 10 mol of water, amounts of the various constituents at the
beginning (t = 0) and completion (t) of the reaction are given as under.
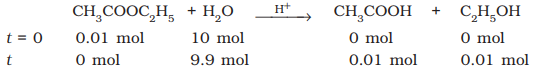
The concentration of water does
not get altered much during the course of the reaction.
So, in the rate equation
Rate = k′
[CH3COOC2H5] [H2O]
the term [H2O]
can be taken as constant.
The equation, thus, becomes
Rate = k [CH3COOC2H5]
where k = k′
[H2O] and the reaction behaves as first order reaction.
A reaction in which concentration
of one of the reactants can be taken as constant and can be included in the
rate constant, are called pseudo first order reactions.
For first order chemical reaction
of type,
![]()
![]()
where Vo,
Vt, and V∞ are the
volumes of NaOH solution used for the titration of
same volume of the reaction mixture after times 0, t and ∞ respectively.
Inversion of cane sugar is
another pseudo first order reaction.
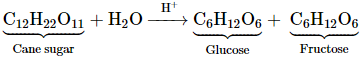
Rate = k [C12H22O11]
Also
![]()
where ro, rt, and
r∞ are the polarimetric
readings (angle of rotation of polarized light) at t = 0, t and ∞,
respectively.
Comparison
of zero and first order rections