Catalysis Homogeneous and Heterogeneous
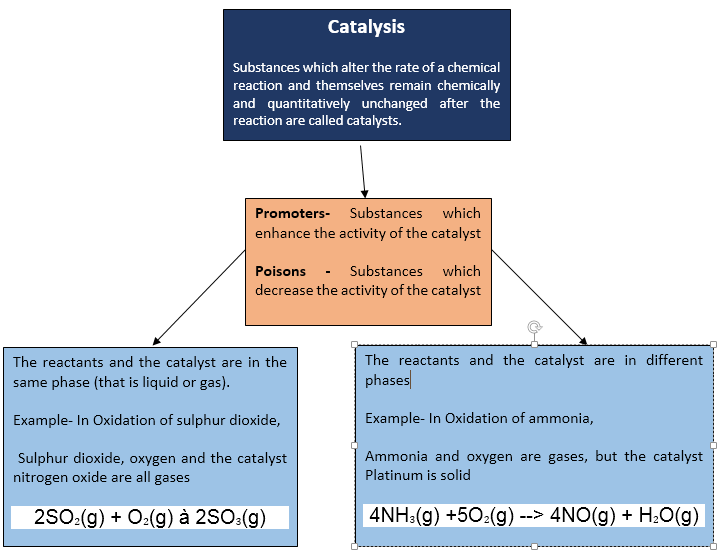
Catalysis:
Catalysis
is the increase in
the rate of
a chemical reaction due to the participation of an additional
substance called a catalyst.
Example:
∑
Potassium
chlorate, when heated strongly decomposes slowly giving dioxygen.
2KClO3 → 2KCl + 3O2
∑
when a
little of manganese dioxide is added, the decomposition takes place at a
considerably lower temperature range
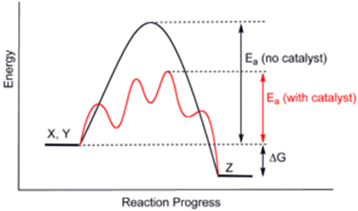
Catalyst:
Substances, which accelerate the rate of a chemical reaction
and themselves remain chemically and quantitatively unchanged after the
reaction, are known as catalysts
∑
The
phenomenon is known as catalysis.
Promoters and Poisons:
Promoters:
Promoters are substances that enhance the
activity of a catalyst
∑
By itself the promoter
has little or no catalytic effect
∑
Some promoters interact
with active components of catalyst and thereby alter their chemical
effect on the catalyzed substance
∑
Commonly used promoters
are metallic ions incorporated into metals and metallic oxide catalysts
Example:
In Haberís process for manufacture of ammonia,
molybdenum acts as a promoter for iron which is used as a catalyst.
N2 (g) + 3H2 (g) ![]() 2NH3 (g)
2NH3 (g)
Poisons:
The substances which decrease the
activity of catalyst are called catalytic poisons or inhibitors
∑
Catalyst
poisoning refers to the partial or total deactivation of a catalyst caused by
exposure to a range of chemical compounds
∑
Poisoning
refers specifically to chemical deactivation, rather than other mechanism of
catalyst degradation such as thermal decomposition or physical damage.
∑
Poisoning
involves compounds which bonds chemically to the active surface sites of a
catalyst
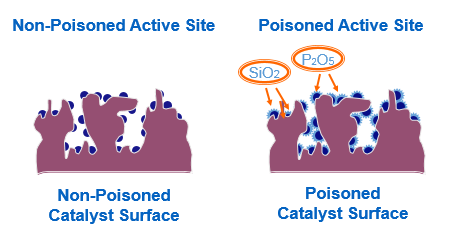
Example:
Arsenic acts as catalytic poison in
the manufacture of sulphuric acid by Ďcontact process.
Types of Catalysis:
1.
Homogeneous
catalysis
2.
Heterogeneous
catalysis
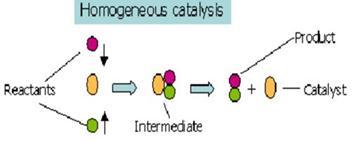
Homogenous Catalysis:
When
the reactants and the catalyst are in the same phase the process is said to be
homogeneous catalysis.
∑
Homogeneous catalysis applies
to reactions in the gas phase and even in solids
∑
Heterogeneous
catalysis is the alternative to homogeneous catalysis
∑
The term is used almost
exclusively to describe solutions and often implies catalysis by organomettalic.
Example:
1.
Hydrolysis
of methyl acetate is catalysed by H+ ions furnished by hydrochloric acid.
CH3COOCH3 (l) + H2 O
(l) HCI (l) CH3COOH (aq) + CH3OH
(aq)
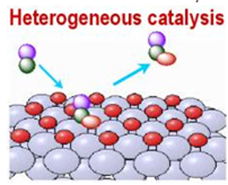
Heterogeneous Catalysis:
The catalytic process in which the reactants and the
catalyst are in different phases is known as heterogeneous catalysis.
∑
This
may refer to phase-solid, liquid and gas
∑
Heterogeneous
catalysts can be more easily recycled than homogeneous catalyst
∑
Characterization
of the catalyst and optimization of properties can be more difficult
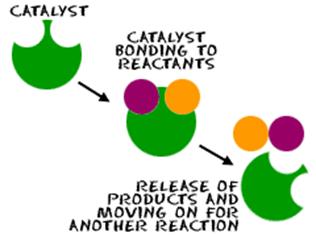
Adsorption Theory of Heterogeneous Catalysis:†††
Adsorption theory explains the mechanism of
heterogeneous catalysis.
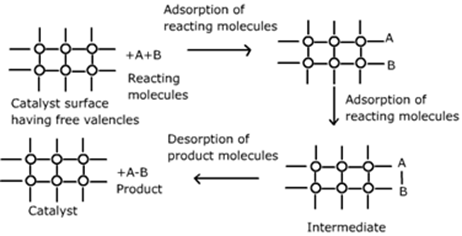
The mechanism involves five steps:
∑
Diffusion
of reactants characteristics of catalyst
∑
†Adsorption of reactant molecules on the
surface of the catalyst.
∑
Occurrence
of chemical reaction on the catalystís surface through formation of an
intermediate
∑
Desorption
of reaction products from the catalyst surface, and thereby, making the surface
available again for more reaction to occur.
∑
Diffusion
of reaction products away from the catalystís surface.
Advantage and Disadvantage of Catalysis:
|
Property |
Homogeneous |
Heterogeneous |
|
Catalyst recovery |
Difficult and
expensive |
Easy and cheap |
|
Thermal stability |
Poor |
good |
|
Selectivity |
Good or
excellent-single active site |
Good or poor-multiple
active |