Colloids
A colloid is a heterogeneous system in which one substance is dispersed
(dispersed phase) as very fine particles in another substance called dispersion
medium.
The essential difference between a solution and a colloid is that of particle
size. While in a solution, the constituent particles are ions or small
molecules, in a colloid, the dispersed phase may consist of particles of a
single macromolecule (such as protein or synthetic polymer) or an aggregate of
many atoms, ions or molecules.
Colloidal
particles are larger than simple molecules but small enough to remain
suspended. Their range of diameters is between 1 and 1000 nm (10–9 to
10–6 m).
Colloidal particles have an enormous surface area per unit mass as a
result of their small size.
This enormous surface area leads to some special properties of colloids.
Classification of colloids
Colloids
are classified on the basis of the following criteria:
i.
Physical
state of dispersed phase and dispersion medium
ii.
Nature of
interaction between dispersed phase and dispersion medium
iii.
Type of
particles of the dispersed phase.
Classification
of colloids based on physical state of dispersed phase and dispersion medium
Sols are solids in liquids, gels are
liquids in solids and emulsions are liquids in liquids.
Types of colloidal systems
|
Dispersed phase |
Dispersion medium |
Type of colloid |
Examples |
|
Solid |
Solid |
Solid sol |
Some coloured glasses and gem stones |
|
Solid |
Liquid |
Sol |
Paints, cell
fluids |
|
Solid |
Gas |
Aerosol |
Smoke, dust |
|
Liquid |
Solid |
Gel |
Cheese, butter,
jellies |
|
Liquid |
Liquid |
Emulsion |
Milk, hair cream |
|
Liquid |
Gas |
Aerosol |
Fog, mist,
cloud, insecticide sprays |
|
Gas |
Solid |
Solid sol |
Pumice stone, foam rubber |
|
Gas |
Liquid |
Foam |
Froth, whipped
cream, soap lather |
Classification of colloids based on nature of
interaction between dispersed phase and dispersion medium
Lyophilic colloids
The word ‘lyophilic’ means solvent attracting or liquid-loving.
If water is the dispersion medium, the term used is hydrophilic. Colloidal
sols directly formed by mixing substances like gum, gelatine, starch, rubber,
etc., with a suitable liquid (the dispersion medium) are called lyophilic sols.
These sols can be reconstituted by simply remixingwith
the dispersion medium. That is why these sols are also called reversible
sols. These sols are quite stable and cannot be easily coagulated.
Lyophobic colloids
The word ‘lyophobic’ means solvent repelling or liquid-hating.
If water is the dispersion medium, the term used is hydrophobic. Substances
like metals, their sulphides, etc., when simply mixed with the dispersion
medium do not form the colloidal sol. Their colloidal sols can be prepared only
by special methods. These sols are readily precipitated (or coagulated)
on the addition of small amounts of electrolytes, by heating
or by shaking and hence, are not stable. Further, once
precipitated, they do not give back the colloidal sol by simple
addition of the dispersion medium. Hence, these sols are also called irreversible
sols. Lyophobic sols need stabilizing agents for their
preservation.
|
Lyophilic Colloids (liquid loving) |
Lyophobic colloids(liquid hating) |
|
Some
substances which can from colloids directly on mixing them with a suitable liquid
(dispersion medium). |
Some substances cannot form
colloid just by directly mixing them with a liquid. Their colloidal sols are
prepared by special methods and are called lyophobic colloids. |
|
Examples
of these substances are gum, gelatin, starch, rubber. |
Examples of these substances are metals, metal sulphides. |
|
They
are also called Reversible sols as in these sols (colloids) when the
dispersion phase is separated from the dispersion medium (by say evaporation),
the sol can be formed again by just mixing the dispersion phase and medium
again. |
They are also called Irreversible
colloids as on precipitation, they don’t give back the colloid on simply mixing the dispersed phase
and the dispersed medium. |
|
They
are also very stable and cannot be coagulated |
They are unstable and coagulate easily by heating shaking
or adding electrolytes. Stabilizing agents are used to preserve them |
Classification of colloids based on type of
particles of the dispersed phase
Multi-molecular colloids
On dissolution, a large number of atoms or smaller molecules of a
substance aggregate together to form species having size in
the colloidal range (diameter < 1 nm). For example,
a gold sol may contain particles of various sizes having many
atoms. Sulphur sol consists of particles containing a thousand
or more of S8 sulphur molecules.
Macromolecular colloids
Macromolecules in
suitable solvents form solutions in which the size of the macromolecules may be
in the colloidal range. Such systems are called macromolecular
colloids. These colloids are quite stable and resemble true solutions in many
respects. Examples are starch, cellulose, proteins, enzymes,
polythene, nylon, polystyrene, synthetic rubber, etc.
Associated colloids (Micelles)
There are some substances which at low concentrations behave as normal
strong electrolytes, but at higher concentrations exhibit colloidal behaviour
due to the formation of aggregates. The aggregated particles thus formed are
called micelles. These are also known as associated
colloids.
The formation of micelles takes place only above a particular
temperature called Kraft temperature (Tk)
and above a particular concentration called critical micelle
concentration (CMC). On dilution, these colloids revert back to individual
ions.
Surface active agents such
as soaps and synthetic detergents belong to this class. For
soaps, the CMC is 10–4 to 10–3 mol L–1. These colloids have both
lyophobic and lyophilic parts. Micelles may contain as many as 100
molecules or more.
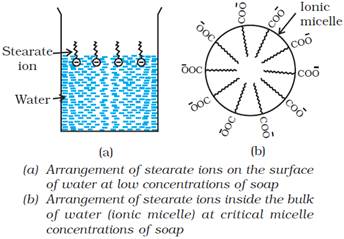
Mechanism of micelle formation
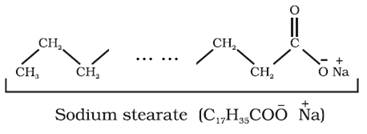
Soap is sodium or potassium salt of a higher fatty acid and may be
represented as RCOO–Na+ (e.g., sodium
stearate CH3(CH2)16COO–Na+,
which is a major component of many bar soaps). When dissolved in water, it
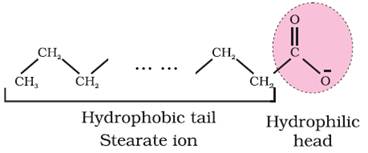
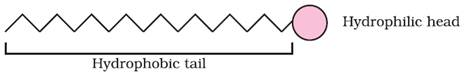
dissociates into RCOO– and Na+ ions. The RCOO– ions,
however, consist of two parts — a long hydrocarbon chain R (also called non-polar
‘tail’) which is hydrophobic (water repelling), and a polar group COO– (also
called polar-ionic ‘head’), which is hydrophilic (water loving).
Cleansing action of soaps
The cleansing action of soap is due to the fact that soap molecules form micelle around
the oil droplet in such a way that hydrophobic part
of the stearate ions is in the oil droplet and hydrophilic part
projects out of the grease droplet like the bristles. Since the polar groups
can interact with water, the oil droplet surrounded by stearate ions is now
pulled in water and removed from the dirty surface. Thus soap helps in emulsification and
washing away of oils and fats. The negatively charged sheath
around the globules prevents them from coming together and
forming aggregates.