Emulsions:
Liquid-Liquid Colloids
“The colloidal systems in which fine droplets of one liquid are
dispersed in another liquid are called emulsions, the two liquids
otherwise being mutually immiscible.”
If a mixture of two immiscible or partially miscible liquids is shaken,
a coarse dispersion of one liquid in the other is obtained which is
called emulsion.
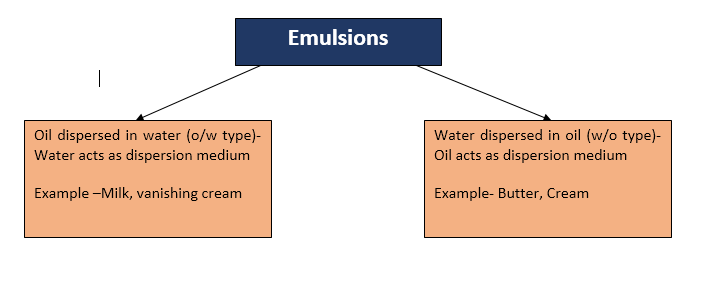
Types of emulsion
Depending
upon the nature of the dispersed phase, the emulsions are classified as;
i.
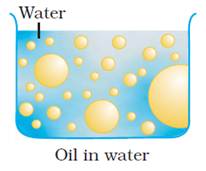
Oil-in-water emulsions (O/W):
The
emulsion in which oil is present as the dispersed phase and water as the
dispersion medium (continuous phase) is called an oil-in-water
emulsion. Milk and vanishing cream are examples of the oil-in-water type of
emulsion. In milk liquid fat globules are dispersed in water.
ii.
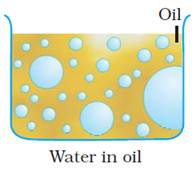
Water-in-oil emulsion (W/O):
The
emulsion in which water forms the dispersed phase, and the oil acts as the
dispersion medium is called a water-in-oil emulsion. These emulsions are also
termed oil emulsions. Butter, cream and cod liver oil, are
examples of this type of emulsion.
Properties of emulsion
- Emulsions of oil in water are unstable and
sometimes they separate into two layers on standing. For stabilisation of
an emulsion, a third component called emulsifying agent is added. The
emulsifying agent forms an interfacial film between suspended particles
and the medium. The principal emulsifying agents for O/W emulsions are
proteins, gums, natural and synthetic soaps, etc., and for W/O, heavy
metal salts of fatty acids, long chain alcohols, lampblack, etc.
- Emulsions can be diluted with
any amount of the dispersion medium. On the other hand, the dispersed
liquid when mixed forms a separate layer.
- The droplets in emulsions are
often negatively charged and can be precipitated by
electrolytes, containing polyvalent metal ions indicating the negative
charge on the globules.
- Emulsions show all the characteristic
properties of colloidal solution such as Brownian movement, Tyndall
effect, electrophoresis etc.
- Emulsions can be broken into constituent
liquids by heating, freezing, centrifuging, etc. This process is also
known as demulsification.
- The size of the dispersed particles in
emulsions is larger than those in the sols. It ranges from 1000 Å to 10,000
Å. However, the size is smaller than the particles in suspensioins.