Ammonia (NH3)
Ammonia is produced in the
atmosphere by decay of urea,
NH2CONH2 +
2H2O → (NH4)2CO3 ↔
2NH3 + H2O + CO2
Preparation
of ammonia
a. Lab method
2NH4Cl
+ Ca (OH)2 →
2NH3 + 2H2O + CaCl2
(NH4)2SO4 +
2NaOH → 2NH3 + 2H2O + Na2SO4
b. Haber’s process
Ammonia is
prepared at high pressure (200 atm) and 700 K, in the
presence of catalysts.
N2 (g) + 3H2 (g) ![]() 2NH3
(g); ΔfH = – 46.1 kJ mol–1
2NH3
(g); ΔfH = – 46.1 kJ mol–1

The optimum conditions for
manufacturing ammonia are:
i.
Pressure (around 200 × 105 Pa)
ii.
Temperature (700 K)
iii.
Catalyst such as iron oxide with
small amounts of Al2O3 and K2O
Properties
of ammonia
(i) Physical properties
of ammonia
Ammonia is a
colourless gas with a pungent odour. Its freezing and boiling
points are 198.4 and 239.7 K respectively. In the solid and liquid states, it
is associated through hydrogen bonds as in the case of water and that accounts
for its higher melting and boiling points than expected on the basis of its
molecular mass.
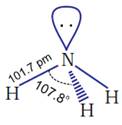
(ii) Geometry of ammonia molecule
The molecule
is trigonal pyramidal with the nitrogen atom at the
apex. It has three bond pairs and one lone pair of electrons.
(iii)
Solubility of ammonia
It is
extremely soluble in water due to H–bonding. Its aqueous solution is weakly
basic due to the formation of OH–ions.
NH3 (g)
+ H2O (l) → NH4+ (aq) + OH- (aq)
(iv) Reaction of ammonia with acids
It forms
ammonium salts with acids, e.g., NH4Cl, (NH4)2SO4,
etc. As a weak base, it precipitates the hydroxides of many metals from their
salt solutions. For example,
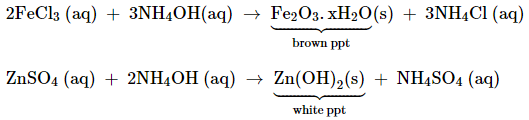
Due to
presence of a lone pair of electrons on the nitrogen atom of the ammonia
molecule, it behaves as a Lewis base. It donates the electron pair and forms
linkage with metal ions. Formation of complex compounds is used for detection
of metal ions such as Cu2+, Ag+:
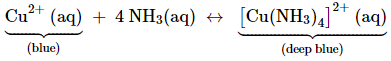
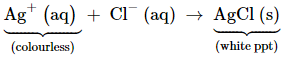
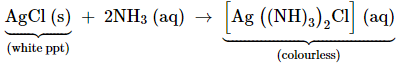
(v)
Reaction of ammonia with chlorine
When NH3 is in
excess, N2 is the main product
8NH3 +
3Cl2 → 6NH4CI + N2
When Cl2 is in
excess, NCl3 is the main product
NH3 +
3Cl2 → NCl3 + 3HCl
Uses
of ammonia
i.
Ammonia is used to produce
various nitrogenous fertilizers (ammonium nitrate, urea, ammonium phosphate and
ammonium sulphate) and in the manufacture of some inorganic nitrogen compounds,
like nitric acid.
ii.
Liquid ammonia is also used as a
refrigerant.