Nitric Acid
(HNO3)
Nitrogen
forms oxoacids such as,
·
H2N2O2 (hyponitrous acid),
·
HNO2 (nitrous
acid)
·
HNO3 (nitric
acid)
Acids
containing oxygen and/or –OH group are called oxoacids.
|
Oxy
Acids |
Name of
oxy – acid |
|
H2N2O2 |
Hyponitrous acid |
|
H2 NO2 |
Hydro-nitrous acid |
|
HNO2 |
Nitrous acid |
|
HNO3 |
Nitric acid |
|
HNO4 |
Per nitric acid |
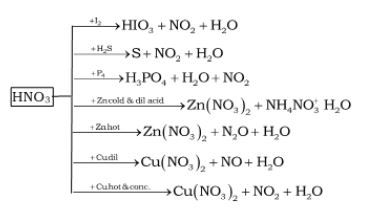
Preparations of HNO3
i.
Lab method (heating of KNO3 or NaNO3 with
conc H2SO4)
NaNO3 + H2SO4 (conc) → NaHSO4 + HNO3
ii.
Ostwald’s process

2NO (g) + O2 (g) ⇔ 2NO2 (g)
3NO2 (g) + H2O (l)
→ 2HNO3 (g) + NO (g)
NO formed
here is recycled.
The aqueous HNO3 can
be concentrated by distillation upto ~ 68% by mass.
Further concentration to 98% can be achieved by dehydration with concentrated H2SO4.
Physical properties of HNO3
It is a syrupy, colourless, pungent liquid
usually available as 68%. 15.7 M aqueous solution is often yellow due to small
concentrations of NO2. At concentration the specific gravity is
1.504.
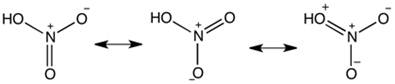
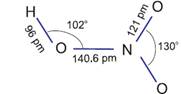
Structure of HNO3
In the gaseous state, HNO3 exists
as a planar molecule with the structure as shown.
Chemical Reactions of HNO3
Acidic character of HNO3
In aqueous solution, nitric acid behaves as
a strong acid giving hydronium and nitrate ions.
HNO3 (aq)
+ H2O (l) → H3O+ (aq)
+ NO3- (aq)
Reactions of
HNO3 with metals
Concentrated nitric acid is a strong oxidising
agent and attacks most metals except noble metals such as gold and
platinum. The products of oxidation depend upon the concentration of the acid,
temperature and the nature of the material undergoing oxidation.
3Cu + 8HNO3 (dilute) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3 (conc.) → Cu(NO3)2 + 2NO2 +
2H2O
4Zn + 10HNO3 (dilute) →
4Zn(NO3)2 + 5H2O
+ N2O
Zn + 4HNO3 (conc.) → Zn(NO3)2 + 2H2O + 2NO2
Some metals (e.g., Cr, Al) do not dissolve in concentrated nitric acid
because of the formation of a passive film of oxide on the surface.
Reaction of HNO3 with non-metals
Concentrated nitric acid also oxidises
non–metals and their compounds. Iodine is oxidised to iodic acid, carbon to
carbon dioxide, sulphur to H2SO4, and phosphorus to
phosphoric acid.
I2 + 10HNO3 (conc.)
→ 2HIO3 + 10NO2 + 4H2O
C + 4HNO3 (conc.) → CO2 +
2H2O + 4NO2
S8 + 48HNO3 (conc.)
→ 8H2SO4 + 48NO2 + 16H2O
P4 + 20HNO3 (conc.)
→ 4H3PO4 + 20 NO2 + 4H2O
Brown ring test of nitrate
Fe2+ reduces nitrates to
nitric oxide, which reacts with Fe2+ to form a brown coloured
complex. Dilute ferrous sulphate solution is added to an
aqueous solution containing nitrate ion, and then adding concentrated sulphuric
acid along the sides of the test tube. A brown ring at the interface between
the solution and sulphuric acid layers indicates the presence of nitrate ion in
solution.
FeSO4 + 6H2O →
[Fe(H2O)6]SO4
2HNO3 + 3H2SO4 +
6FeSO4 → 3Fe2(SO4)3 +
2NO + 4H2O
[Fe(H2O)6]SO4+
NO → [Fe(H2O)5(NO)]SO4 +
H2O
or
NO3- + 3Fe2+ +
4H+ → NO + 3Fe3+ + 2H2O
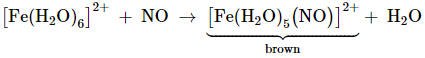
Uses of nitric acid
1.
Used in
the manufacture of ammonium nitrate for fertilisers and other nitrates for use
in explosives and pyrotechnics.
2.
Used for
the preparation of nitroglycerin, trinitrotoluene and
other organic nitro compounds.
3.
Used in
the pickling of stainless steel, etching of metals and as an
oxidiser in rocket fuels.
4.
Used as
nitrating reagent.