Group 18 Elements
Ø The 18th group of the Periodic Table consists of
colourless, odourless gases at room temperature.
Ø Group-18 elements constitute the last group of p-block elements and include Helium
(He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn).
Ø All these are gases and chemically
unreactive. They form very few compounds, because of this they are termed as
noble gases.
Ø
Helium
is an exception as it does not have p-orbitals.
All the elements in the group are gases.
Occurrence
All these gases except radon and oganesson occur in the atmosphere. Their atmospheric
abundance in dry air is ~ 1% by volume of which argon is the major constituent.
Helium and sometimes neon are found
in minerals of radioactive origin e.g., pitchblende, monazite, cleveite. The
main commercial source of helium is natural gas.
Xenon and radon are the rarest
elements of the group. Radon is obtained as a decay product of 226Ra.
![]() +
+
![]() →
→
![]() +
3n
+
3n
Oganesson has been synthetically produced by
collision of ![]() atoms and
atoms and ![]() ions
ions
Electronic configuration
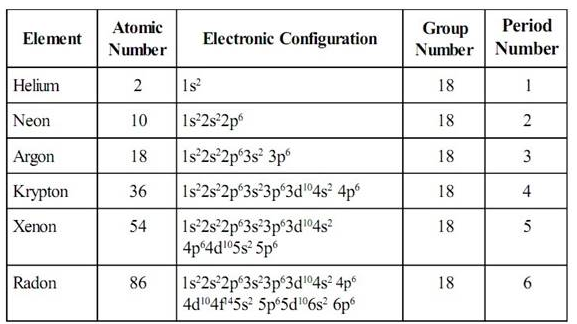
Their valence shell electronic configuration
is ns2np2 except helium which has 1s2.
The properties of noble gases including their inactive nature are ascribed to
their closed shell structures.