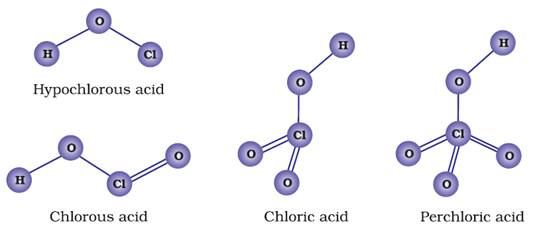
Oxoacids of Halogens
Ø
Due to high electronegativity and
small size and absence of d-orbitals; fluorine forms only one oxoacid, HOF known as fluoric (I) acid or hypofluorous acid.
Ø
The other halogens form several oxoacids. Most of them cannot be isolated in pure state.
They are stable only in aqueous solutions or in the form of their salts.
Ø
+3 oxidation state of bromine and
iodine are unstable due to inert pair effect, therefore, HBrO2 and
HIO2 do not exist.
Ø
The central atom in the oxoacids is sp3 hybridized. Every oxoacid has essentially one X-OH bond. Whereas most oxoacids have X = O bonds present in them. This double bond
between oxygen and halogen is dπ-pπ in nature.
Ø
In the series of oxoacids, the first member possesses high acidic strength.
This is due to high electronegativity and small size of the halogen atom. The
acidic strength increases with increase in the oxidation number of halogens.
|
Halic (I) acid (Hypohalous) |
HOF (Hypofluorous) |
HOCl (Hypochlorous) |
HOBr (Hypobromous) |
HOI (Hypoiodous) |
|
Halic (III)
acid (Halous acid) |
– |
HOCIO (chlorous
acid) |
– |
– |
|
Halic (V)
acid (Halic acid) |
– |
HOCIO2 (chloric acid) |
HOBrO2 (bromic acid) |
HOIO2 (iodic acid) |
|
Halic (VII)
acid (Perhalic acid) |
– |
HOCIO3 (perchloric
acid) |
HOBrO3 (perbromic
acid) |
HOIO3 (periodic acid) |