Oxygen and
Its Compounds
Dioxygen
Preparation of dioxygen
- By heating oxygen containing salts such
as chlorates, nitrates and permanganates.
2KClO3 ![]() 2KCl +
3O2
2KCl +
3O2
- By the thermal decomposition of the
oxides of metals low in the electrochemical series and higher oxides of
some metals.
2Ag2O (s) → 4Ag (s) + O2
(g)
2HgO (s) → 2Hg (l) + O2 (g)
2Pb3O4 (s) → 6PbO
(s) + O2 (g)
2PbO2 (s) → 2PbO (s) + O2
(g)
- Hydrogen peroxide readily decomposes
into water and dioxygen by catalysts such as
finely divided metals and manganese dioxide.
2H2O2 (aq) → 2H2O (l) + O2 (g)
- On large scale it can be prepared from
water or air. Electrolysis of water leads to the release
of hydrogen at the cathode and oxygen at the anode.
Industrially, dioxygen is
obtained from air by first removing carbon dioxide and water vapour and then,
the remaining gases are liquefied and fractionally
distilled to give dinitrogen
and dioxygen.
Physical
properties of dioxygen
It is colourless, odourless, tasteless,
slightly heavier than air and sparingly soluble in water. It liquefies at 90 K
and freezes at 55 K. Oxygen atom has three stable isotopes: 16O, 17O
and 18O. Molecular oxygen, O2 is unique in
being paramagnetic inspite of having
even number of electrons.
Chemical
properties of dioxygen
Dioxygen directly reacts with nearly all metals and
non-metals except some metals (e.g., Au, Pt) and some
noble gases. In many cases one element forms two or more oxides.
Its combination with other elements is
strongly exothermic which helps in sustaining the reaction. However, to
initiate the reaction, some external heating is required as bond dissociation
enthalpy of oxgyen-oxygen double bond is high (493.4
kJ mol–1).
2Ca + O2 → 2CaO
4Al + 3O2 → 2Al2O3
P4 + 5O2 →
P4O10
C + O2 → 2CO2
2ZnS + 3O2 → 2ZnO + 2SO2
CH4 + 2O2 →
CO2 + 2H2O
Some compounds are catalytically oxidized,
2SO2 + O2 ![]() 2SO3
2SO3
4HCl + O2 ![]() 2Cl2 + 2H2O
2Cl2 + 2H2O
Uses of dioxygen
Ø In respiration and combustion
Ø In welding and cutting using oxy-hydrogen or
oxy-acetylene torch
Ø In iron and steel industry to increase the
content of blast
Ø In life support systems e.g., in hospitals,
for divers, miners and mountaineers
Ø In combustion of rocket fuels, e.g., hydrazines in liquid oxygen, which provides tremendous
thrust in rockets.
Tests of O2
With NO it gives reddish brown fumes of NO2.
It is adsorbed by alkaline pyrogallol and the
solution turns brown.
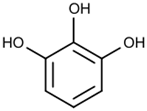
(pyrogalol)
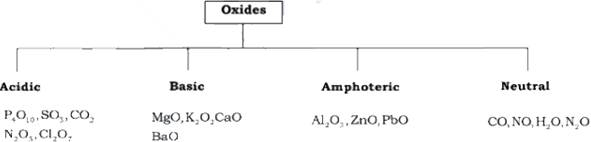
The oxides vary widely in their nature and
properties. Oxides can be simple (e.g., MgO, Al2O3)
or mixed (Pb3O4, Fe3O4).
Simple oxides can be classified on the basis
of their acidic, basic or amphoteric character. An oxide that combines with
water to give an acid is termed acidic oxide (e.g., SO2, Cl2O7,
CO2, N2O5). For
example, SO2 combines with water to give H2SO3,
an acid.
SO2 + H2O →
H2SO3
As a general rule, only non-metal
oxides are acidic but oxides of some metals in
high oxidation state also have acidic character (e.g., Mn2O7,
CrO3, V2O5).
The oxides which give a base with water are
known as basic oxides (e.g., Na2O, CaO, BaO). For example, CaO combines with water to give Ca(OH)2.
CaO + H2O → Ca(OH)2
In general, metallic oxides are basic.
Metallic oxides which exhibit characteristics of both acidic as well as basic
oxide, are known as amphoteric oxides. They react with acids
as well as alkalies.
Al2O3(s) + 6HCl (aq) + 9H2O(l) → 2
[Al(H2O)6]3+ (aq)
+ 6Cl- (aq)
Al2O3(s) + 6NaOH (aq) + 3H2O(l) →
2Na3[(Al(OH)6] (aq)
The oxides which are neither acidic nor basic are known as neutral
oxides. Examples are CO, NO and N2O.