Ozone (O3)
Ozone is an allotropic form of oxygen. It is
too reactive to remain for long in the atmosphere at sea level. It is formed
from atmospheric oxygen in the presence of sunlight at a height of about 20
kilometres,. This ozone layer protects the earth’s
surface from an excessive concentration of ultraviolet (UV) radiations.
Preparation
of ozone
By passing a slow dry stream of oxygen
through a silent electrical discharge, oxygen gets converted to ozone (10%).
The product is known as ozonised oxygen.
3O2 → 2O3, ΔHo (298 K) = +142 kJ mol–1
Since the formation of ozone from oxygen is
an endothermic process, it is necessary to use a silent
electrical discharge in its preparation to prevent its
decomposition.
If concentration of ozone greater than 10 per
cent is required, a battery of ozonisers can be used, and pure ozone (b.p. 385 K) can be condensed in a
vessel surrounded by liquid oxygen.
Physical
properties of ozone
Pure ozone is a pale blue gas, dark blue
liquid and violet-black solid. Ozone has a characteristic smell and in small
concentrations it is harmless. However, if the concentration rises above 100
parts per million, breathing becomes uncomfortable resulting in headache and
nausea.
Chemical reactions of ozone
(i) Decomposition of
ozone
Ozone is thermodynamically unstable with respect to oxygen since its
decomposition into oxygen results in the liberation of heat (ΔH is
negative) and an increase in entropy (ΔS is positive). These two effects
reinforce each other, resulting in large negative Gibbs energy change (ΔG)
for its conversion into oxygen. Therefore, high concentrations of ozone can be
dangerously explosive.
2O3 → 3O2;
ΔH = -284 kJ/mol
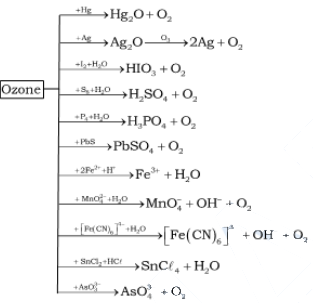
(ii) Oxidising action
Due to the ease with which it liberates atoms
of nascent oxygen (O3 → O2 + [O]), it
acts as a powerful oxidising agent.
PbS(s) + 4O3(g)
→ PbSO4(s) + 4O2(g)
2FeSO4 + H2SO4 +
O3 → Fe2(SO4)2 +
H2O + O2
It liberates iodine from neutral KI solution
and the liberated I2, turns starch paper blue.
2KI (aq)
+ H2O(l) + O3(g) → 2KOH(aq) + I2(s) + O2(g)
I2 + Starch → Blue
Colour
Nitrogen oxides (particularly nitric oxide) combine very rapidly with
ozone and there is, thus, the possibility that nitrogen oxides emitted from the
exhaust systems of supersonic jet aeroplanes might be slowly depleting the
concentration of the ozone layer in the upper atmosphere.
NO(g) + O3(g) → NO2 (g)
+ O2 (g)
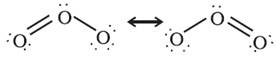
(iii) Resonance of O3
The two oxygen-oxygen bond lengths in the
ozone molecule are identical (128 pm) and the molecule is angular with a bond
angle of about 117o.
Uses of ozone
Ø As a germicide and disinfectant for
sterilizing water.
Ø As a bleaching agent for oils, ivory wax and
delicate fibres.
Ø For detecting, the position of double bond in
unsaturated compounds.
Ø In destroying odours coming from cold storage
room, slaughter houses and kitchen of hotels.
Ø In the manufacture of potassium permanganate.