Thermodynamics
Introduction:
What is
Thermodynamics?
·
Thermodynamics is the branch of physics that deals with
the concepts of heat and temperature and the inter conversion of heat and other
forms of energy.
·
Thermodynamics is a macroscopic science. It deals with
bulk systems and does not go into the molecular constitution of matter.
·
Thermodynamic description involves relatively few
macroscopic variables of the system, which are suggested by common sense and can
be usually measured directly.
·
Example: Would involve specifying the co-ordinates and
velocities of the huge number of molecules constituting the gas.
Thermodynamics vs Mechanics:
·
In Thermodynamics
only the state of the object which means only consider macroscopic variables
like pressure, volume and temperature.
·
In mechanics
consider the motion, velocity and acceleration of the object.
·
Example: In mechanics if a bullet is fired from a gun
we will consider the motion of bullet and its velocity, acceleration etc.
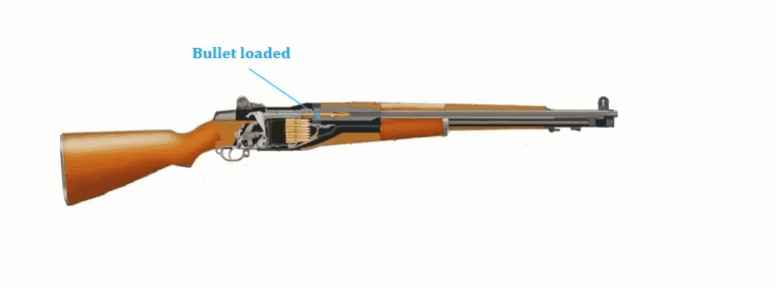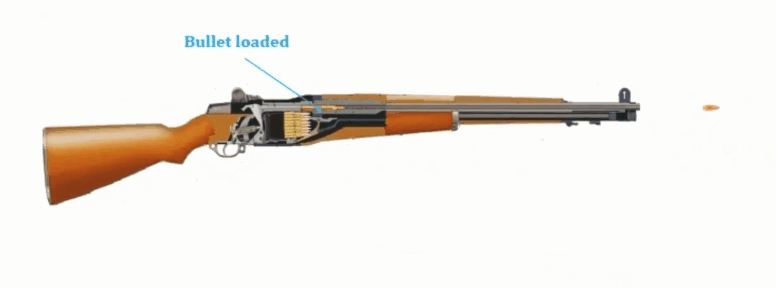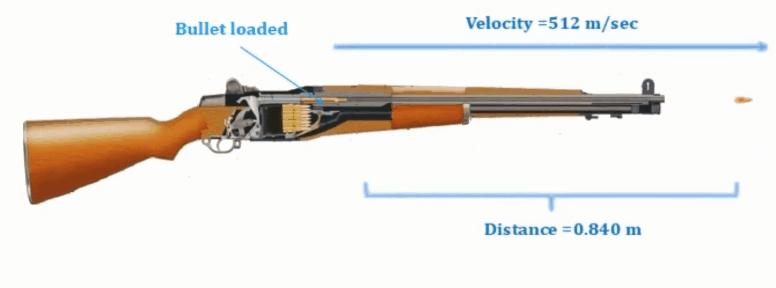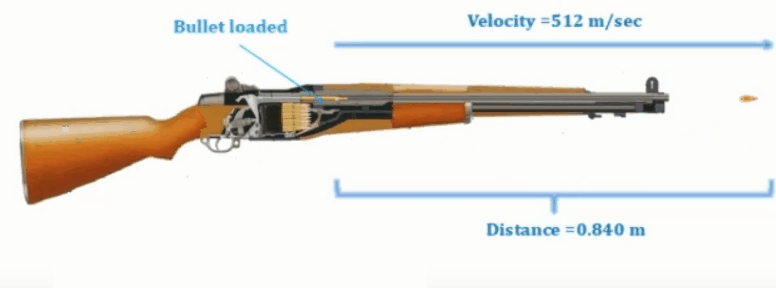

Bullet getting
fired from gun
Consider a second scenario where bullet is
fired on a wall. When it hits the wall its kinetic energy gets converted to
heat. Due to heat generation there will be a change in temperature and the
internal energy of the bullet. These are the macroscopic variables in
Thermodynamics.

Bullet fired from
the gun and hitting a wall
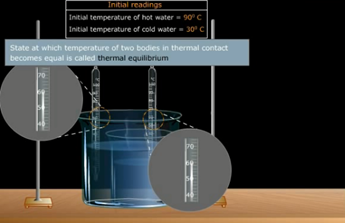
Thermal Equilibrium:
What is Thermal Equilibrium?
Two systems are said to be in thermal
equilibrium with each other if they have the same temperature.
Thermal
Equilibrium
How it is Attained?
Consider a gas inside
a closed rigid container, completely insulated from its surroundings, with
fixed values of pressure, volume, temperature, mass and composition that do not
change with time, is in a state of thermodynamic equilibrium.
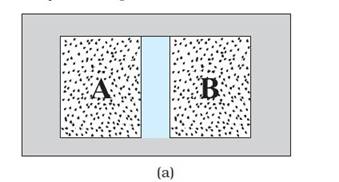
Consider two gases A and B occupying two
different containers. Pressure and
volume of a given mass of gas can be chosen to be its two independent
variables.
Let the pressure and volume of the gases be (PA, VA) and (PB, VB) respectively.
Suppose first that the two systems are put in proximity but are separated by an adiabatic wall – an insulating wall
(can be movable) that does not allow flow of energy (heat) from one to another.
The systems are insulated from the rest of the surroundings also by similar
adiabatic walls.
Systems A and B (two gases) separated by an adiabatic
wall – an insulating wall that does not allow flow of heat.
In figure A:
It is found that any possible pair of values (PA, VA) will be in
equilibrium with any possible pair of values (PB, VB).
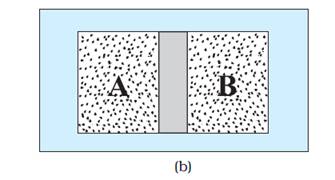
The same systems A and B separated by
a diathermic wall – a conducting wall that allows heat to flow from one to
another. In this case, thermal equilibrium is attained in due course.
In figure B:
Suppose that the adiabatic wall is replaced by a diathermic wall – a conducting wall
that allows energy flow (heat) from one to another. It is then found that the
macroscopic variables of the systems A and B change spontaneously
until both the systems attain equilibrium states. After that there is no change
in their states.
Conclusion:
The pressure and volume variables of the two
gases change to (PB , VB ) and (PA , VA ) such
that the new states of A and B are in
equilibrium with each other .There is no more energy flow from one to another
.We then say that the system A is in
thermal equilibrium with the system B.
Definition of Temperature (Zeroth Law of
Thermodynamics):
What is Zeroth Law of Thermodynamics?
This law
identifies thermal equilibrium and introduces temperature as a tool for
identifying equilibrium.
“Zeroth law of thermodynamics states that when
two systems are in thermal equilibrium through a third system separately then
they are in thermal equilibrium with each other also”.
Example for Zeroth Law of Thermodynamics:
·
Consider two
systems A and B which are separated by an adiabatic wall. Heat flow happens
between systems A and C, and between B and C, due to which all 3 systems attain
thermal equilibrium.
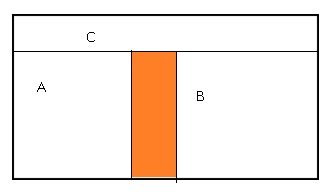
·
Systems A and B
are in thermal equilibrium with C. Then they will be in equilibrium with each
other also.
·
Zeroth Law of
Thermodynamics suggested that there should be some physical quantity which
should have same value for the system to be in thermal equilibrium.
·
This physical
quantity which determines whether system is in equilibrium or not
is Temperature.
·
Temperature is the
quantity which determines whether the system is in thermal equilibrium with the
neighboring system.
·
When the
temperature becomes equal then the flow of heat stops.
How does Zeroth Law of Thermodynamics leads to the
Concept of Temperature?
Concept of
Temperature: Temperature is a physical quantity which has the same
value for all systems which are in thermal equilibrium with each other.
Temperature of a system determines whether it is in thermal equilibrium or not
with another system.
·
There exists a scalar quantity called
"temperature" which is a property of all thermodynamic system such
that temperature equality is a necessary and sufficient condition for thermal
equilibrium.
·
Heat
may be defined as energy in transit.
·
Word
heat is used only if there is a transfer of energy from one thermodynamic
system to another.
·
When
two systems at different temperatures are kept in contact with each other then,
after some time temperatures of both the systems become equal and this
phenomenon can be described by saying that energy has flown from one system to
another.
·
This
flow of energy from one system to another on account of temperature difference
is called heat transfer.
·
Flow
of heat is a non-mechanical mode of energy transfer.
·
Heat
flow depends not only on initial and find states but also on path it's.