Heat, Work and Internal Energy
Internal Energy:
What is
meant by Internal Energy of a System?
The
internal energy of a system is the sum of molecular kinetic and potential
energies in the frame relative to which the centre of
mass of the system is at rest. The intermolecular potential energy of a real
gas is a function of its volume. The internal kinetic energy of a gas is a
function of its temperature.
The
kinetic energy does not include overall kinetic energy of the system .It
includes only the disordered energy associated with random motion of the
molecules. It is denoted as “U”. Internal
energy can be described as the sum of kinetic and potential energies of
individual molecules in the material. But in thermodynamics one should keep in
mind that “U” is simply a macroscopic variable of the system.
“U” is thermodynamic state
variable and its value depends only on the given state of the system and not on
path taken to arrive the state.
Transfer of heat and performance of work are
two mean of adding or subtracting energy from a system. On transfer of energy,
system is said to have undergone a change in internal energy. Thus the sum of
heat put into the system plus work done on the system equals increase in
internal energy of the system for any process. Here heat and work are the state
1 and state 2 of internal energy.
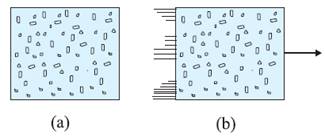
a) Internal energy U of a gas is the sum of
the kinetic and potential energies of its molecules when the box is at rest.
Kinetic energy due to various types of motion
(translational, rotational, vibrational) is
to be included in U. (b) If the same box is moving as a whole with some
velocity, the kinetic energy of the box is not to be included in U.
If U1 is internal energy of
state 1 and U2 is internal energy of state 2 than change in
internal energy would be
ΔU = U2 - U1 ------ (1)
If W is the work done by the system on its
surroundings then -W would be the work done on the system by the surroundings.
If Q is the heat put into the system then,
Q +
(-W) = ΔU ------
(2)
usually written as
Q = ΔU+W
------
(3)
Equation (3) is known as first law of
thermodynamics and it can be applied when Q, W and U are
expressed in same units.
Some Important stuffs are given as follows:
(1) Q is positive when heat is given
to the system and Q is negative when heat is taken from the system
(2) W is positive when system
expands and does work on surroundings
Hence when a certain amount of heat Q
is given to the system then some part of it is used in increasing internal
energy ΔU of the system while remaining part leaves the system in
form of work done by the system on its surroundings. From equation (3) it is
observed that first law of thermodynamics is a statement of conservation of
energy stated as: ' The energy put into the system equals the sum of the work
done by the system and the change in internal energy of the system'
If the system undergoes any process in
which ΔU = 0 i.e., charge in internal energy is zero then from
equation (3)
Q = W
that is heat supplied to the system is used up
entirely in doing work on the surroundings. Stability and nature of the
internal energy of an ideal gas and system:
·
Internal
energy of a system is a thermodynamic “stable variable”
·
Internal
energy of an ideal gas is purely “kinetic in nature”.
Two Ways of Changing the Internal Energy of
a System:
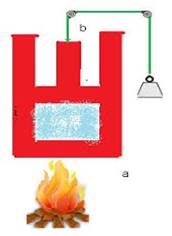
Heat and work are two distinct modes of
energy transfer to a system that results in change in its internal energy. (a) Heat
is energy transfer due to temperature difference between the system and the
surroundings. (b) Work is energy
transfer brought about by means (e.g. moving the piston by raising or lowering
some weight connected to it) that do not involve such a temperature difference.
The system to be a certain mass of gas contained in a cylinder with a
movable piston. This shows there are two ways of changing the state of the gas
(and hence its internal energy). One way is to put the cylinder in contact with
a body at a higher temperature than that of the gas. The temperature difference
will cause a flow of energy (heat) from the hotter body to the gas, thus
increasing the internal energy of the gas. The other way is to push the piston
down i.e. to do work on the system, which again results in increasing the
internal energy of the gas. Both these things could happen in the reverse
direction. With surroundings at a lower temperature, heat would flow from the
gas to the surroundings. Likewise, the gas could push the piston up and do work
on the surroundings.
Hence heat and work are two different modes of altering the state of a
thermodynamic system and changing its internal energy. The notion of heat
should be carefully distinguished from the notion of internal energy. Heat is
certainly energy, but it is the energy in transit. This is not just a play of
words. The distinction is of basic significance. The state of a thermodynamic
system is characterized by its internal energy, not heat. A statement like ‘a gas in a given state
has a certain amount of heat’ is as meaningless as the statement that ‘a gas in a given
state has a certain amount of work’. In contrast, ‘a gas in a given state has a certain amount of internal
energy’ is a perfectly meaningful statement. Similarly, the statements ‘a certain amount of
heat is supplied to the system’ or ‘a certain amount of work was done by the
system’ are perfectly meaningful.
Are Heat and Work State Variable?
Heat
and work in thermodynamics are not state variables. They are modes of energy
transfer to a system resulting in change in its internal energy, which, as already mentioned, is
a state variable. In ordinary language, we often confuse heat with internal
energy. The distinction between them is sometimes ignored in elementary physics
books. For proper understanding of thermodynamics, however, the distinction is
crucial.
Sign Conversion used in the Measurement of
Heat, Work and Internal energy:
·
Heat
absorbed by the system is positive and heat given out by the system is
negative.
·
Work
done by a system is positive and work done on a system is negative.
·
The
increase in internal energy of a system is positive and the decrease in
internal energy of a system is negative.
Difference between Heat and Work:
|
Heat |
Work |
|
It is a mode of energy
transfer due to temperature difference between the system and the
surroundings. |
Work is the mode of energy transfer
brought about by means that do not involve temperature difference. |
|
When heat is supplied
to a gas, its molecule move faster in all direction at random. |
When a piston compresses a gas to do work
on it, it forces the molecule to move in the direction of piston’s motion. |
|
So heat is a mode of
energy transfer that produces random motion. |
So work may be regarded as the mode of
energy transfer that produces organized motion. |
Heat (ΔQ):
It is the energy that is transferred between a system and
its environment.
1.
Heat is a form of
energy so it is a scalar quantity with dimension (ML2T–2).
2.
Unit: Joule
(S.I.), Calorie (1 calorie = 4.2 Joule)
3.
Heat is a path
dependent quantity.
4.
ΔQ = mL [For change in state] and ΔQ = mcΔT [For change in temperature]
5.
(ΔQ) = μCvΔT [For constant volume] and (ΔQ) p = μCpΔT [For constant pressure]
Work (ΔW):
Work done ΔW =![]()
= ![]()
= P (![]() )
)
1.
Like heat, work is
also a path dependent, scalar physical quantity with dimension (ML2T–2).
2.
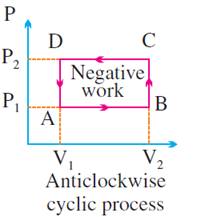
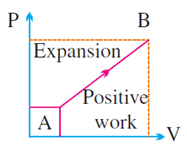
ΔW = positive if Vf > Vi i.e., system expands
ΔW = negative if Vf < Vi i.e., system contracts
3.
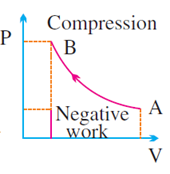
W = area under P – V
diagram
·
It is positive if
volume increases (for expansion)
·
It is negative if
volume decreases (for compression)
·
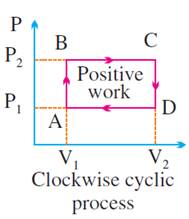
It is positive if
the cycle is clockwise.
·
It is negative if
the cycle is anticlockwise.