Nuclear Force
The average mass nuclei the
binding energy per nucleon is approximately 8 MeV, which is much larger than
the binding energy in atoms.
Therefore, to bind a
nucleus together there must be a strong attractive force of a totally different
kind.
This force is nuclear force
(strongest force in nature). It is strong enough to overcome the repulsion
between the (positively charged) protons and to bind both protons and neutrons
into the tiny nuclear volume.
Some important features of
the nuclear binding force are given below:
(i) The nuclear force is much
stronger than the Coulomb force acting between charges or the gravitational
forces between masses. The nuclear binding force has to dominate over the
Coulomb repulsive force between protons inside the nucleus. This happens only
because the nuclear force is much stronger than the Coulomb force. The
gravitational force is much weaker than even Coulomb force.
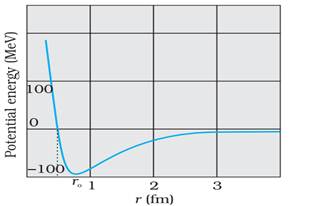
(ii)
The nuclear force between two nucleons falls rapidly to zero as their distance
is more than a few femtometres. This leads to saturation of forces in a medium
or a large-sized nucleus, which is the reason for the constancy of the binding
energy per nucleon. A rough plot of the potential energy between two nucleons
as a function of distance is shown in the figure given below. The potential
energy is a minimum at a distance r of about 0.8 fm. This means that the
force is attractive for distances larger than 0.8 fm and repulsive if they are
separated by distances less than 0.8 fm.
(iii)
The nuclear force between neutron-neutron, proton-neutron and proton-proton is
approximately the same. The nuclear force does not depend on the electric
charge.