Conformations of Ethane
Alkanes contain carbon-carbon sigma (σ)
bonds. Electron distribution of the sigma molecular orbital is symmetrical
around the internuclear axis of the C–C bond which is
not disturbed due to rotation about its axis. This permits free rotation about
C–C single bond. This rotation results into different spatial arrangements of
atoms in space which can change into one another. Such spatial arrangements of
atoms which can be converted into one another by rotation around a C-C single
bond are called conformations or conformers or rotamers.
Alkanes can thus have infinite number of
conformations by rotation around C-C single bonds. However, it may be
remembered that rotation around a C-C single bond is not completely free. It is
hindered by a small energy barrier of 1-20 kJ mol–1
due to weak repulsive interaction between the adjacent bonds. Such a type of
repulsive interaction is called torsional strain.
Conformations of Ethane
Ø Ethane molecule (C2H6)
contains a carbon – carbon single bond with each carbon atom attached to three
hydrogen atoms.
Ø Considering the ball and stick model
of ethane, keep one carbon atom stationary and rotate the other carbon atom
around the C-C axis. This rotation results into infinite number of spatial
arrangements of hydrogen atoms attached to one carbon atom with respect to the
hydrogen atoms attached to the other carbon atom. These are called
conformational isomers (conformers).
Ø Thus there are infinite number of
conformations of ethane. However, there are two extreme cases.
Ø One such conformation in which
hydrogen atoms attached to two carbons are as closed together as possible is
called eclipsed conformation and the other in which hydrogens are as far apart
as possible is known as the staggered conformation.
Ø Any other intermediate conformation
is called a skew conformation.
Ø It may be remembered that in all the
conformations, the bond angles and the bond lengths remain the same.
Ø Eclipsed and the staggered
conformations can be represented by
o
Sawhorse
projections
o
Newman
projections
Sawhorse Projections
Ø In this projection, the molecule is
viewed along the molecular axis.
Ø It is then projected on paper by
drawing the central C–C bond as a somewhat longer straight line.
Ø Upper end of the line is slightly
tilted towards right or left hand side.
Ø The front carbon is shown at the
lower end of the line, whereas the rear carbon is shown at the upper end.
Ø Each carbon has three lines attached
to it corresponding to three hydrogen atoms.
Ø The lines are inclined at an angle
of 120° to each other.
Sawhorse projections of eclipsed and staggered conformations of ethane
are depicted in below figure.
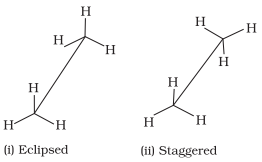
Sawhorse
projections of ethane
Newman Projections
Ø In this projection, the molecule is
viewed at the C–C bond head on.
Ø The carbon atom nearer to the eye is
represented by a point.
Ø Three hydrogen atoms attached to the
front carbon atom are shown by three lines drawn at an angle of 120° to each
other.
Ø The rear carbon atom (the carbon
atom away from the eye) is represented by a circle and the three hydrogen atoms
are shown attached to it by the shorter lines drawn at an angle of 120° to each
other.
The Newman’s projections are depicted in below figure.
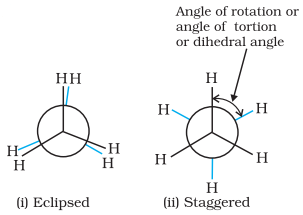
Newman’s
projections of ethane
Relative stability of conformations
Ø In staggered form of ethane, the
electron clouds of carbon-hydrogen bonds are as far apart as possible.
Ø Thus, there are minimum repulsive
forces, minimum energy and maximum stability of the molecule.
Ø On the other hand, when the staggered
form changes into the eclipsed form, the electron clouds of the carbon –
hydrogen bonds come closer to each other resulting in increase in electron
cloud repulsions.
Ø To check the increased repulsive
forces, molecule will have to possess more energy and thus has lesser
stability.
Ø The repulsive interaction between
the electron clouds, which affects stability of a conformation, is called
torsional strain.
Ø Magnitude of torsional strain
depends upon the angle of rotation about C–C bond. This angle is also called
dihedral angle or torsional angle.
Ø Of all the conformations of ethane,
the staggered form has the least torsional strain and the eclipsed form, the
maximum torsional strain.
Ø Therefore, staggered conformation is
more stable than the eclipsed conformation.
Ø Hence, molecule largely remains in
staggered conformation or we can say that it is preferred conformation.
Ø Thus it may be inferred that
rotation around C–C bond in ethane is not completely free.
Ø The energy difference between the
two extreme forms is of the order of 12.5 kJ mol–1,
which is very small.
Ø Even at ordinary temperatures, the
ethane molecule gains thermal or kinetic energy sufficient enough to overcome
this energy barrier of 12.5 kJ mol–1
through intermolecular collisions.
Ø Thus, it can be said that rotation
about carbon-carbon single bond in ethane is almost free for all practical
purposes.
Ø It has not been possible to separate
and isolate different conformational isomers of ethane.