Preparation
Alkenes belong
to the family of hydrocarbons. They contain a double bond between the carbon
atoms. Alkenes preparation can be done by various methods. Explore different
methods of preparation of alkenes.
From alkynes:
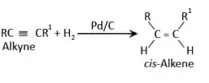
Alkynes are used for
the preparation. Alkyne to alkene conversion is carried out by the reduction of
alkynes with hydrogen in the presence palladised
charcoal. The charcoal used is moderately deactivated with the help of quinoline or sulphur compounds.
This reaction results in the formation of alkenes. Palladised
charcoal which is halfway deactivated is called as Lindlarís
catalyst. The alkenes obtained from the above reaction have cis geometry. In
order to form trans alkenes, alkynes are made to
undergo reduction with sodium in liquid ammonia.
From alcohols:
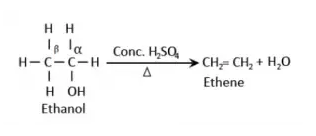
Alcohols reacts with
concentrated sulphuric acid which results in the
formation of alkenes due to the elimination of a water molecule. As water
molecule is removed in this reaction, it is called as acidic dehydration of
alcohol and the dehydrating agent is concentrated sulphuric
acid.
From vicinal halides:
Vicinal dihalides can be defined as the dihalides
in which two adjacent carbon atoms are attached to two halogens. When such dihalides react with zinc metal, they lose halogen
molecules which result in the formation of alkenes. Such a reaction of
preparation of alkenes from Vicinal dihalides is known as dehalogenation
![]()