Particulate Pollutants
Particulates pollutants are the minute solid particles or liquid droplets in air. These are present in vehicle emissions, smoke particles from fires, dust particles and ash from industries. Particulates in the atmosphere may be viable or non-viable. The viable particulates e.g., bacteria, fungi, moulds, algae etc., are minute living organisms that are dispersed in the atmosphere. Human beings are allergic to some of the fungi found in air. They can also cause plant diseases.
Non-viable particulates may be classified according to their nature and size as follows:
a) Smoke particulates consist of solid or mixture of solid and liquid particles formed during combustion of organic matter. Examples are cigarette smoke, smoke from burning of fossil fuel, garbage and dry leaves, oil smoke etc.
b) Dust is composed of fine solid particles (over 1µm in diameter), produced during crushing, grinding and attribution of solid materials. Sand from sand blasting, saw dust from wood works, pulverized coal, cement and fly ash from factories, dust storms etc., are some typical examples of this type of particulate emission.
c) Mists
are produced by particles of spray liquids and by condensation of vapours in
air. Examples are sulphuric acid mist and herbicides and insecticides that miss
their targets and travel through air and form mists.
d) (d)
Fumes are generally obtained by the condensation of vapours during sublimation,
distillation, boiling and several other chemical reactions. Generally, organic
solvents, metals and metallic oxides form fume particles.
The effect of
particulate pollutants are largely dependent on the particle size. Airborne
particles such as dust, fumes, mist etc., are dangerous for human health.
Particulate pollutants bigger than 5 microns are likely to lodge in the nasal
passage, whereas particles of about 10 micron enter into lungs easily. Lead
used to be a major air pollutant emitted by vehicles. Leaded petrol used to be
the primary source of air-borne lead emission in Indian cities. This problem
has now been overcome by using unleaded petrol in most of the cities in India.
Lead interferes with the development and maturation of red blood cells.
Smog
The word smog is derived from smoke and fog. This is the most common example of air pollution that occurs in many cities throughout the world. There are two types of smog:
a) Classical smog occurs in cool humid climate. It is a mixture of smoke, fog and sulphur dioxide. Chemically it is a reducing mixture and so it is also called as reducing smog.
b) Photochemical smog occurs in warm, dry and sunny climate. The main components of the photochemical smog result from the action of sunlight on unsaturated hydrocarbons and nitrogen oxides produced by automobiles and factories. Photochemical smog has high concentration of oxidising agents and is, therefore, called as oxidising smog.
Formation
of photochemical smog
When fossil fuels are burnt, a variety of pollutants are emitted into the earth’s troposphere. Two of the pollutants that are emitted are hydrocarbons (unburnt fuels) and nitric oxide (NO). When these pollutants build up to sufficiently high levels, a chain reaction occurs from their interaction with sunlight in which NO is converted into nitrogen dioxide (NO2). This NO2 in turn absorbs energy from sunlight and breaks up into nitric oxide and free oxygen atom (below figure).
Photochemical smog occurs where
sunlight acts on vehicle pollutants
NO2(g) ![]() NO(g) + O(g) ------ (i)
NO(g) + O(g) ------ (i)
Oxygen atoms are very reactive and combine with the O2 in air to produce ozone.
O(g) + O2(g) ⇌ O3(g) ------ (ii)

The ozone formed in the above reaction (ii) reacts rapidly with the NO(g) formed in the reaction (i) to regenerate NO2 . NO2 is a brown gas and at sufficiently high levels can contribute to haze.
NO(g) + O3(g) → NO2(g) + O2(g) ------ (iii)
Ozone is a toxic gas and both NO2 and O3 are strong oxidising agents and can react with the unburnt hydrocarbons in the polluted air to produce chemicals such as formaldehyde, acrolein and peroxyacetyl nitrate (PAN).
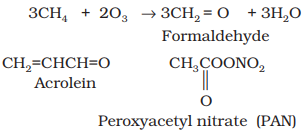
Effects of photochemical smog
Ø The common components of photochemical smog are ozone, nitric oxide, acrolein, formaldehyde and peroxyacetyl nitrate (PAN).
Ø Photochemical smog causes serious health problems.
Ø Both ozone and PAN act as powerful eye irritants.
Ø Ozone and nitric oxide irritate the nose and throat and their high concentration causes headache, chest pain, dryness of the throat, cough and difficulty in breathing.
Ø Photochemical smog leads to cracking of rubber and extensive damage to plant life.
Ø It also causes corrosion of metals, stones, building materials, rubber and painted surfaces.
How can photochemical smog be controlled ?
Ø Many techniques are used to control or reduce the formation of photochemical smog.
Ø If we control the primary precursors of photochemical smog, such as NO2 and hydrocarbons, the secondary precursors such as ozone and PAN, the photochemical smog will automatically be reduced.
Ø Usually catalytic converters are used in the automobiles, which prevent the release of nitrogen oxide and hydrocarbons to the atmosphere.
Ø Certain plants e.g., Pinus, Juniparus, Quercus, Pyrus and Vitis can metabolise nitrogen oxide and therefore, their plantation could help in this matter.