Expressing Concentration of Solutions
Concentration of Solution
The Concentration of a Solution
is defined as the relative amount of solute present in a solution. It basically
talks about how to find the amount of solute present in solvent which together
forms solution.
There
are several ways by which we can describe the concentration of the solution
quantitatively.
(i) Mass
percentage (w/w):
The mass percentage of a component of a
solution is defined as:
Mass %
of a component = ![]()
Example:
If a solution is described by 10%
glucose in water by mass, it means that 10 g of glucose is dissolved in 90 g of
water resulting in a 100 g solution. Concentration described by mass percentage
is commonly used in industrial chemical applications. For example, commercial
bleaching solution contains 3.62 mass percentage of sodium hypochlorite in
water.
(ii)
Volume percentage (V/V):
The volume percentage is defined as:
Volume %
of a component = ![]()
Example:
10% ethanol solution in
water means that 10 mL of ethanol is dissolved in water such that the total
volume of the solution is 100 mL. Solutions
containing liquids are commonly expressed in this unit. For example, a 35% (![]() /
/![]() ) solution of ethylene glycol, an antifreeze, is
used in cars for cooling the engine. At this concentration the antifreeze
lowers the freezing point of water to 255.4K (–17.6°C).
) solution of ethylene glycol, an antifreeze, is
used in cars for cooling the engine. At this concentration the antifreeze
lowers the freezing point of water to 255.4K (–17.6°C).
(iii)
Mass by volume percentage (w/V):
Another unit which is commonly used in
medicine and pharmacy is mass by volume percentage. It is the mass of solute dissolved in 100 mL of the
solution.
(iv) Parts per million:
When a solute is present in trace quantities, it is convenient to express
concentration in parts per
million (ppm) and is
defined as:
Parts
per million = ![]()
As in
the case of percentage, concentration in parts per million can also be
expressed as mass to mass, volume to volume and mass to volume. A litre of sea
water (which weighs 1030 g) contains about 6 × 10–3 g of dissolved
oxygen (O2). Such a small concentration is also expressed as 5.8 g
per 106 g (5.8 ppm) of sea water. The concentration of pollutants in
water or atmosphere is often expressed in terms of µg mL–1 or ppm.
(v)
Mole fraction:
Commonly used symbol for mole fraction is ![]() and subscript used on the right hand side of
and subscript used on the right hand side of ![]() denotes the component. It is defined as:
denotes the component. It is defined as:
Mole
fraction of a component = ![]()
Example:
In a binary mixture, if the number of moles of A
and B are nA and nB respectively, the
mole fraction of A will be
![]() A
=
A
= ![]()
For a solution containing i
number of components, we have:
![]() =
= ![]()
= ![]()
It can be shown that in a given solution sum of
all the mole fractions is unity, i.e.
![]() = 1
= 1
Mole fraction unit is very useful in relating
some physical properties of solutions, say vapour pressure with the
concentration of the solution and quite useful in describing the calculations
involving gas mixtures.
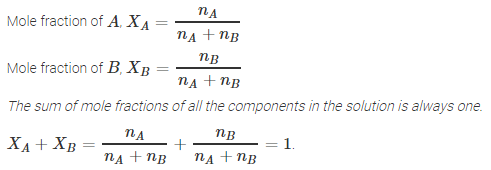
(vi)
Molarity:
Molarity (M) is defined as number of moles of solute
dissolved in one litre (or one cubic decimetre) of solution,
Molarity = ![]()

Example:
0.25 mol L–1
(or 0.25 M) solution of NaOH means that 0.25 mol of NaOH has been dissolved in
one litre (or one cubic decimetre).
(vii) Molality:
Molality (m) is defined as the number of moles of the
solute per kilogram (kg) of the solvent and is expressed as:
Molality (m) = ![]()
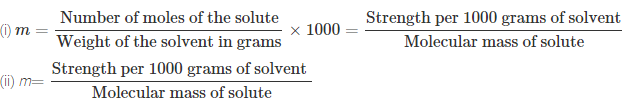
Example:
1.00 mol
kg–1 (or 1.00 m) solution of KCl means
that 1 mol (74.5 g) of KCl
is dissolved in 1 kg of water.