Solubility
Solubility
of a substance is its maximum amount that can be dissolved in a specified
amount of solvent at a specified temperature. It depends upon the nature of
solute and solvent as well as temperature and pressure.
Solubility of a Solid in a
Liquid
Ø It is
observed that polar solutes dissolve in polar solvents and non-polar solutes in
nonpolar solvents.
Ø A solute
dissolves in a solvent if the intermolecular interactions are similar in the
two.
Dissolution: When a
solid solute is added to the solvent, some solute dissolves and its
concentration increases in solution. This process is known as dissolution.
Crystallisation: Some
solute particles in solution collide with the solid solute particles and get
separated out of solution. This process is known as crystallisation.
Solute +
Solvent ⇌ Solution ------ (1)
A stage is reached when the two processes
occur at the same rate. Under such conditions, number of solute particles going
into solution will be equal to the solute particles separating out and a state
of dynamic equilibrium is reached. At this stage the concentration of solute in
solution will remain constant under the given conditions, i.e., temperature and
pressure.
Saturated solution: A solution
in which no more solute can be dissolved at the same temperature and pressure
is called a saturated solution.
Unsaturated solution: An unsaturated solution is one in
which more solute can be dissolved at the same temperature.
Effect of temperature
Ø The
solubility of a solid in a liquid is significantly affected by temperature
changes. Consider the
equilibrium represented by equation (1). This, being dynamic equilibrium, must
follow Le Chateliers Principle.
Ø If in a nearly saturated solution, the dissolution
process is endothermic (Δsol H >
0), the solubility should increase with rise in temperature and if it is
exothermic (Δsol H < 0) the
solubility should decrease.
Effect of pressure
Ø Pressure
does not have any significant effect on solubility of solids in liquids. It is
so because solids and liquids are highly incompressible and practically remain
unaffected by changes in pressure.
Solubility of a Gas in Liquid
Factors
Affecting Solubility of Gases in Liquids:
Nature of gas and nature of solvent:
Ø
The gases which are easily liquefiable are relatively more soluble than
dihydrogen and dioxygen.
Ø
The gases which are capable of undergoing a chemical reaction with the
water are relatively more soluble in water than other solvents.
Effect of temperature:
Ø
The solubility of gases in liquids decreases with the rise in
temperature. When dissolved, the gas molecules are present in the liquid phase
and the process of dissolution can be considered similar to condensation and
heat is evolved in this process.
Ø
We know that dissolution process involves dynamic equilibrium and thus
must follow Le Chatelier’s Principle. As dissolution
is an exothermic process, the solubility should decrease with the increase of
temperature.
Effect of pressure:
Ø
The solubility of gases in liquids is greatly affected by pressure and
temperature. The solubility of gases increases with the increase of pressure.
Ø
For a solution of gases in a solvent, consider a system as shown in
below figure (a). The lower part is the solution and the upper part is the
gaseous system at pressure p and temperature T. Assume this system to be in a
state of dynamic equilibrium, i.e., under these conditions rate of gaseous
particles entering and leaving the solution phase is the same.
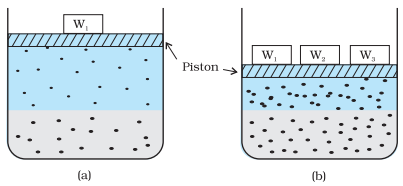
Effect of pressure on the
solubility of a gas. The concentration of dissolved gas is proportional to the
pressure on the gas above the solution.
Ø
Now increase the pressure over the solution phase by compressing the gas
to a smaller volume (above figure (b)). This will increase the number of
gaseous particles per unit volume over the solution and also the rate at which
the gaseous particles are striking the surface of the solution to enter it. The
solubility of the gas will increase until a new equilibrium is reached
resulting in an increase in the pressure of a gas above the solution and thus
its solubility increases.
Henry’s Law:
Henry was the first to give
a quantitative relation between pressure and solubility of a gas in a solvent
which is known as Henry’s law.
The law states that at a
constant temperature, the solubility (S) of a gas in a liquid is directly
proportional to the pressure (P) of the gas.
S α P
∴ S = KP
where K = Henry’s constant
Dalton, a contemporary of
Henry, also concluded independently that the solubility of a gas in a liquid
solution is a function of partial pressure of the gas.
If we use the mole fraction
of a gas in the solution as a measure of its solubility, then it can be said
that the mole fraction of gas in the solution is proportional to the partial
pressure of the gas over the solution. The most commonly used form of Henry’s
law states that “the partial pressure of the gas in the vapour phase (p) is proportional to the mole fraction
of the gas (![]() ) in the solution” and is
expressed as:
) in the solution” and is
expressed as:
p = KH ![]()
where KH is Henry’s law
constant.
If we draw a graph of the
partial pressure of the gas versus mole fraction of the gas in solution, then
we should get a plot of the type as shown.
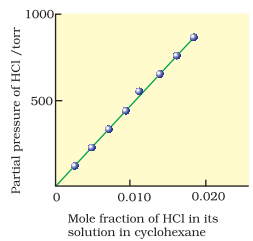
Experimental results for the
solubility of HCl gas in cyclohexane at 293 K. The
slope of the line is the Henry’s Law constant, KH.
Different gases have
different KH values at the same temperature. This suggests that KH
is a function of the nature of the gas. From above equation, we can conclude
that higher the value of KH at a given pressure, the lower is the
solubility of the gas in the liquid.
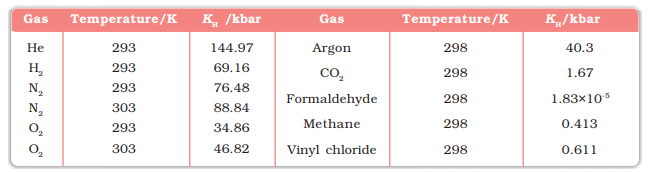
Values of Henry's Law Constant
for Some Selected Gases in Water
Applications
of Henry’s Law:
Henry’s law finds several applications in industry and explains some
biological phenomena.
i.
To increase the solubility of CO2 in soft drinks and soda
water, the bottle is sealed under high pressure.
ii.
Scuba divers must cope with high concentrations of dissolved gases while
breathing air at high pressure underwater. Increased pressure increases the
solubility of atmospheric gases in the blood. When the divers come towards the
surface, the pressure gradually decreases. This releases the dissolved gases
and leads to the formation of bubbles of nitrogen in the blood. This blocks
capillaries and creates a medical condition known as bends, which are painful
and dangerous to the life. To avoid bends, as well as, the toxic effects
of high concentrations of nitrogen in the blood, the tanks used by scuba divers
are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1%
oxygen).
iii.
At high altitudes, the partial pressure of oxygen is less than that at
the ground level. This leads to low concentrations of oxygen in the blood and
tissues of people living at high altitudes or climbers. Low blood oxygen causes
climbers to become weak and unable to think clearly, symptoms of a condition
known as anoxia.
Limitations of Henry’s Law:
Henry’s law is applicable only under following conditions.
1.
The pressure of the gas in not too high.
2.
The temperature is not too low.
3.
The gas should not undergo any chemical reaction with solvent.
4.
The gas should not undergo dissociation in the solvent.