Vapour Pressure of Solutions
Lowering
of Vapour Pressure
Concept of
Vapour Pressure:
Ø If
a container is partially filled with a liquid, a portion of liquid evaporates
to fill the remaining volume of the container with vapour.
Ø The
molecules of liquid evaporated are in continuous random motion, they collide
with the walls of the container and with each other. Thus they create pressure
on the walls of the container and on the liquid. At the same time some
molecules which have left liquid return back to the liquid, the process is
called condensation. After some interval of time, an equilibrium is established
between the two phases of substance. At this stage, the rate of evaporation is
equal to the rate of condensation.
Ø The
pressure exerted by the vapours of the liquid on the surface of the liquid when
equilibrium is established between liquid and its vapour is called vapour
pressure of the liquid.
Ø The
temperature at which vapour pressure of the liquid is equal to the external
pressure is called boiling temperature at that pressure.
Lowering of Vapour Pressure:
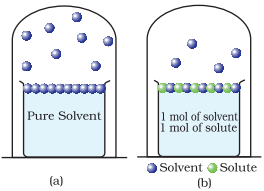
Decrease
in the vapour pressure of the solvent on account of the presence of solute in
the solvent (a) evaporation of the molecules of the solvent from its surface is
denoted by blue, (b) in a solution, solute particles have been denoted by green
and they also occupy part of the surface area.
Ø Liquids
at a given temperature vapourize and under
equilibrium conditions, the pressure exerted by the vapours of the liquid over
the liquid phase is called vapour pressure [Figure (a)].
Ø In
a pure liquid, the entire surface is occupied by the molecules of the liquid.
If a non-volatile solute is added to a solvent to give a solution [Figure (b)],
the vapour pressure of the solution is solely from the solvent alone. This
vapour pressure of the solution at a given temperature is found to be lower
than the vapour pressure of the pure solvent at the same temperature.
Ø In
the solution, the surface has both solute and solvent molecules; thereby the
fraction of the surface covered by the solvent molecules gets reduced.
Consequently, the number of solvent molecules escaping from the surface is
correspondingly reduced thus, the vapour pressure is also reduced.
Ø The
decrease in the vapour pressure of solvent depends on the quantity of
non-volatile solute present in the solution, irrespective of its nature.
Relative
Lowering of Vapour Pressure:
The relative lowering of vapour pressure
for the given solution is the ratio of vapour pressure lowering of solvent from
solution to the vapour pressure of the pure solvent.
Mathematically, the relative lowering of vapour pressure is given by
![]() =
= ![]()
where,
![]() = Vapour pressure of pure solvent
= Vapour pressure of pure solvent
Δp =
Lowering of vapour pressure
p
= Vapour pressure of solution