Purification of Colloidal Solutions
Colloidal
solutions when prepared; generally contain excessive amount of electrolytes and
some other soluble impurities. While the presence of traces of electrolyte is
essential for the stability of the colloidal solution, larger quantities
coagulate it. It is, therefore, necessary to reduce the concentration of these
soluble impurities to a requisite minimum.
The process
used for reducing the amount of impurities to a requisite minimum is known as purification of
colloidal solution.
The purification of colloidal
solution is carried out by the following mehods:
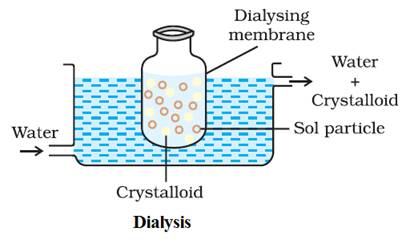
Dialysis:
It is a
process of removing a dissolved substance from a colloidal
solution by means of diffusionthrough
a suitable membrane.
The principle
is based upon the fact that colloidal particles cannot pass through animal
membrane (bladder) or parchment or cellophane
membrane while the ions of the electrolyte can pass through it.
The apparatus used
for this purpose is called dialyser.
A bag of
suitable membrane containing the colloidal solution is suspended in a vessel
through which fresh water is continuously flowing. The impurities slowly
diffused out of the bag leaving behind pure colloidal solution.
The
distilled water is changed frequently to avoid accumulation of the crystalloids
otherwise they may start diffusing back into the bag.
Dialysis can be used for
removing HCl from the ferric hydroxide sol.
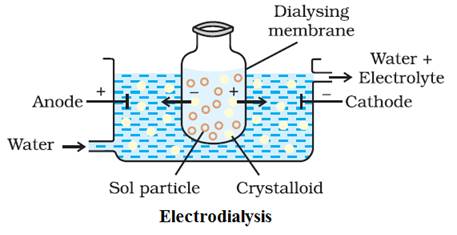
Electro-dialysis:
Ordinarily,
the process of dialysis is quite slow. It can be made faster by applying an
electric field if the dissolved substance in the impure colloidal solution is
only an electrolyte. The process is then named electrodialysis.
The
colloidal solution is placed in a bag of suitable membrane while pure water is
taken outside.
Electrodes
are fitted in the compartment. The ions present in the colloidal solution migrate out
to the oppositely charged electrodes.
Ultra–filtration:
Sol
particles directly pass through ordinary filter paper because their pores are larger
(more than 1μ) than the size of sol particles (less than 200 μ).
If the pores
of the ordinary filter paper are made smaller by soaking the filter paper in a
solution of gelatin of colloidion and subsequently hardened by soaking
in formaldehyde, the treated filter paper may retain colloidal particles
and allow the true solution particles to escape. Such filter paper is known
as ultra-filter and the process of separating colloids by
using ultra – filters is known as ultra-filtration.
Ultra–centrifugation:
The sol
particles are prevented from setting out under the action of gravity by kinetic
impacts of the molecules of the medium.
The setting
force can be enhanced by using high speed centrifugal machines having 15,000 or
more revolutions per minute. Such machines are known as ultra–centrifuges.
Coagulation
or Flocculation or Precipitation
The process
of settling of colloidal particles is called coagulation or
precipitation of the sol.
The
stability of the lyophobic sols is due to the presence of charge on
colloidal particles. If, somehow, the charge is removed, the particles will
come nearer to each other to form aggregates (or coagulate) and settle down
under the force of gravity.
Coagulation
of lyophobic sols
1. By electrophoresis:
The colloidal
particles move towards oppositely charged electrodes, get discharged and
precipitated.
2. By mixing two oppositely charged sols:
When
oppositely charged sols are mixed in almost equal proportions, their charges
are neutralised. Both sols may be partially or completely precipitated. For
example mixing of hydrated ferric oxide (+ve sol) and
arsenious sulphide (–ve
sol) bring them in the precipitated forms. This type of coagulation is
called mutual coagulation or meteral
coagulation.
3. By boiling:
When a sol
is boiled, the adsorbed layer is disturbed due to increased collisions with the
molecules of dispersion medium. This reduces the charge on the particles and
ultimately they settle down to form a precipitate.
4. By persistent dialysis:
On prolonged
dialysis, the traces of the electrolyte present in the sol are removed almost
completely and the colloids become unstable.
5. By addition of electrolytes:
When excess
of an electrolyte is added, the colloidal particles are precipitated. The
reason is that colloids interact with ions carrying charge
opposite to that present on themselves. This causes
neutralization leading to their coagulation.
The ion
responsible for neutralisation of charge on the particles is called the coagulating
ion or flocculating ion. A negative ion causes
the precipitation of positively charged sol and vice versa.
Hardy
schulze rule:
The
coagulation capacity of different electrolytes is different. It depends upon
the valency of the flocculating ion.
According to Hardy Schulze rule,
“greater the valency
of the active ion or flocculating ion, greater will be its coagulating power”
For example to coagulate negative
sol, the coagulation power of cations is in the order Al3+ >
Mg2+ > Na+.
Similarly, to coagulate a positive
sol such as Fe(OH)3,
the coagulating power of different anions is in the order
[Fe(CN)6]4- > PO43- >
SO42- > Cl-
Coagulation
or flocculation value
“The minimum
concentration of an electrolyte which is required to cause the
coagulation or flocculation of a sol is known as flocculation value.”
or “The number of millimoles of an electrolyte
required to bring about the coagulation of one litre of a colloidal solution is
called its flocculation value.”
Coagulation value or flocculating value ∝ 1coagulating power Coagulation value or flocculating value ∝ 1coagulating power.