Allotropes of Sulphur
Sulphur forms many allotropes
of which the yellow
rhombic (α-sulphur) and monoclinic (β-sulphur) forms are
the most important.
The stable form at
room temperature is rhombic
sulphur, which transforms to
monoclinic sulphur when heated above 369 K.
Rhombic sulphur (α-sulphur)
It is yellow in colour with m.p. 385.8 K and specific gravity 2.06. Rhombic sulphur
crystals are formed on evaporating the solution of roll sulphur in CS2. It is
insoluble in water but dissolves to some extent in benzene, alcohol and ether.
It is readily soluble in CS2.
Roll sulphur is
sulphur in the form of rods or sticks made by casting molten sulphur
Monoclinic sulphur (β-sulphur)
Colourless, m.p.
is 393 K and specific gravity 1.98. It is soluble in CS2.
It is prepared by melting
rhombic sulphur in a dish and cooling, till crust is formed. Two holes are made
in the crust and the remaining liquid poured out. On removing the crust,
colourless needle shaped crystals of β-sulphur are formed.
It is stable above 369 K and
transforms into α-sulphur below it. Conversely, α-sulphur is stable
below 369 K and transforms into β-sulphur above this. At 369 K both the
forms are stable. This temperature is called transition temperature.
α-sulphur ![]() β-sulphur
β-sulphur
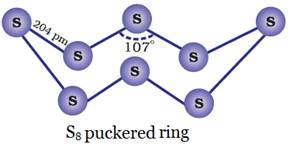
Both rhombic and monoclinic
sulphur have S8 molecules.
These S8 molecules
are packed to give different crystal structures. The S8 ring in both the forms is
puckered and has a crown shape.
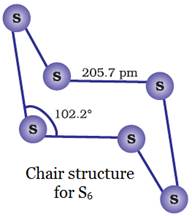
Several other modifications of
sulphur containing 6-20 sulphur atoms per ring have been synthesized. In cyclo-S6, the
ring adopts the chair form.
At elevated temperature (~1000 K), S2 is
the dominant species and is paramagnetic like O2.