First Law of Thermodynamics
What is
First Law of Thermodynamics?
It states
that, “when a certain amount of heat energy is supplied to a system, some part
of it is used to perform external work and rest of heat is used to increase the
internal energy of the system.”
First
Law of Thermodynamics
Derivation
of First Law of Thermodynamics:
Consider the internal energy U of a system can change
through two modes of energy transfer: Heat and Work.
Let ![]() Q = Heat supplied to the system by the surroundings
Q = Heat supplied to the system by the surroundings
![]() W = Work done by the system on the surroundings
W = Work done by the system on the surroundings
![]() U = Change in internal
energy of the system
U = Change in internal
energy of the system
The general principle of conservation of energy then
implies that
![]() Q =
Q = ![]() U +
U + ![]() W ------ (1)
W ------ (1)
i.e. the energy (![]() Q) supplied to the system
goes in partly to increase the internal energy of the system (
Q) supplied to the system
goes in partly to increase the internal energy of the system (![]() U) and the rest in work
on the environment (
U) and the rest in work
on the environment (![]() W). It is simply the
general law of conservation of energy applied to any system in which the energy
transfer from or to the surroundings is taken into account.
W). It is simply the
general law of conservation of energy applied to any system in which the energy
transfer from or to the surroundings is taken into account.
Let us put Eq. (1) in the alternative form
![]() Q –
Q – ![]() W =
W = ![]() U ------ (2)
U ------ (2)
Now, the system may go
from an initial state to the final state in a number of ways. For example, to
change the state of a gas from (P1, V1) to (P2, V2), first change the volume of the gas from V1 to V2, keeping its pressure
constant i.e. first go the state (P1, V2) and then change the pressure of the gas from P1 to P2, keeping volume
constant, to take the gas to (P2, V2). Alternatively, first keep the volume constant and then
keep the pressure constant. Since U is a state variable, ![]() U depends only on the
initial and final states and not on the path taken by the gas to go from one to
the other. Generally
U depends only on the
initial and final states and not on the path taken by the gas to go from one to
the other. Generally ![]() Q and
Q and![]() W will depend on the
path taken to go from the initial to final states.
W will depend on the
path taken to go from the initial to final states.
From the First Law of
Thermodynamics, Eq. (2), it is clear that the combination, ![]() Q –
Q – ![]() W is path independent.
This shows that if a system is taken through a process in which
W is path independent.
This shows that if a system is taken through a process in which ![]() U = 0.
U = 0.
![]() Q =
Q = ![]() W
W
i.e., heat supplied to
the system is used up entirely by the system in doing work on the environment.
If the system is a gas in a cylinder with a movable piston, the gas in moving
the piston does work. Since force is pressure time area, and area time
displacement is volume, work done by the system against a constant pressure P is
|
|
Where ![]() V is the change in
volume of the gas. Thus, for this case, Eq. (1) gives
V is the change in
volume of the gas. Thus, for this case, Eq. (1) gives
![]() Q =
Q = ![]() U + P
U + P ![]() V ------ (3)
V ------ (3)
Change in internal
energy for 1 g of water when go from its liquid to vapour
phase. The measured latent heat of water is 2256 J/g. i.e., for 1 g of water ![]() Q = 2256 J. At atmospheric pressure, 1 g of water has a
volume 1 cm3 in liquid phase and 1671 cm3 in vapour phase.
Q = 2256 J. At atmospheric pressure, 1 g of water has a
volume 1 cm3 in liquid phase and 1671 cm3 in vapour phase.
∴ ![]() W = P (Vg –Vl)
W = P (Vg –Vl)
= 1.013 × 105
× (1671 × 10–6)
= 169.2 J
Equation (3) then
gives
![]() U = 2256 – 169.2
U = 2256 – 169.2
![]() U = 2086.8 J ------ (4)
U = 2086.8 J ------ (4)
Thus most of the heat goes to increase the internal
energy of water in transition from the liquid to the vapour
phase.
Specific
Heat Capacity:
Specific heat is defined as the amount of heat required to raise the
temperature of a body per unit mass.
It depends on:
·
Nature
of substance
·
Temperature
·
Denoted
by ‘s’
Mathematically:
|
s = |
where m = mass of the body
ΔQ = amount of heat absorbed or rejected by the substance
ΔT = temperature change
Unit: J kg–1
K–1
Example: If we are
heating up oil in a pan, more heat is needed when heating up one cup of oil
compared to just one tablespoon of oil. If the mass s is more the amount of
heat required is more to increase the temperature by one degree.
Molar Specific Heat Capacity:
Molar specific heat
capacity of a substance is defined as the amount of heat required to raise the temperature
of one gram molecule of the substance through one degree centigrade. It is
denoted by C. One mole of substance contains M gram of substance where M is the
molecular weight of the substance. So, C = Mc. If n is the number of moles of
substance, then,
n = ![]()
So, m = nM
Substituting for m in equation,
c = ![]()
We get,
Mc = ![]()
Thus,
C = Mc = ![]()
Specific heat of water is taken to be 1. This is because of
the reason for defining unit of heat (calorie) by making use of water.
Heat Capacity or Thermal Capacity:
It is
defined as the amount of heat required to raise the temperature of body through
1°C.
If
ΔT = 1°C, Q = heat capacity = mc
|
Q = |
Thus,
heat capacity of a body is equal to the product of mass and its specific heat
capacity.
Unit: kcal K-1 or JK-1
Specific
Heat Capacity of Water:
The old
unit of heat was calorie. One calorie was earlier defined to be the amount of
heat required to raise the temperature of 1g of water by 1°C. With more precise
measurements, it was found that the specific heat of water varies slightly with
temperature. The following graph shows this variation in the temperature range
0 to 100°C.
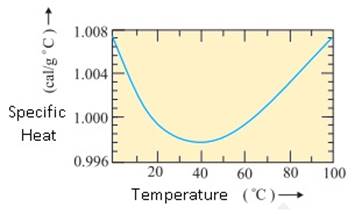
Variation of specific heat capacity of water
with temperature.
For a
precise definition of calorie, it was, necessary to specify the unit
temperature interval. One calorie is defined to be the amount of heat required
to raise the temperature of 1g of water from 14.5 °C to15.5 °C. Since heat is
just a form of energy, it is preferable to use the unit joule, J.
In SI
units, the specific heat capacity of water is 4186 J kg–1 K–1
i.e. 4.186 J g-1 K–1. The so called mechanical equivalent
of heat defined as the amount of work needed to produce 1 cal
of heat is in fact just a conversion factor between two different units of
energy: calorie to joule. Since in SI units, we use the unit joule for
heat, work or any other form of energy, the term mechanical equivalent is now
superfluous and need not be used.
As already
remarked, the specific heat capacity depends on the process or the conditions
under which heat capacity transfer takes place.
Example: For
gases, define two specific heats: specific heat capacity at
constant volume and specific heat capacity at constant pressure. For an
ideal gas, the relation is as follows,
CP
– Cv = R ------
(1)
Where CP and Cv are molar
specific heat capacities of an ideal gas at constant pressure and volume
respectively and R is the universal
gas constant. To prove the relation, begin with the following equation for 1
mole of the gas:
![]() Q =
Q = ![]() U + P
U + P ![]() V
V
If ![]() Q is
absorbed at constant volume,
Q is
absorbed at constant volume, ![]() V = 0
V = 0
Cv = ![]() =
= ![]() =
= ![]() ------ (2)
------ (2)
Where the subscript v is dropped in the last step,
since U of an
ideal gas depends only on temperature.
(The subscript denotes the quantity
kept fixed.) If, ![]() Q is
absorbed at constant pressure,
Q is
absorbed at constant pressure,
CP = ![]() =
= ![]() =
= ![]() ------ (3)
------ (3)
The subscript P can be
dropped from the first term since U of an
ideal gas depends only on T. Now, for
a mole of an ideal gas, which gives
PV = RT
P![]() = R ------
(4)
= R ------
(4)
Equations (2) to (4) give the desired
relation, Eq. (1).
Example: Water has highest specific heat of
capacity because of which it is used as a coolant in automobile radiators and
in hot water bags.
Hot water bag

Water in radiator of the car
Thermodynamic
Processes:
Some special thermodynamic
processes:
|
Type of Processes |
Features |
|
Isothermal |
Temperature
constant |
|
Isobaric |
Pressure constant |
|
Isochoric |
Volume
constant |
|
Adiabatic |
No heat flow between the
system and the surroundings ( |
Comparison
between Isothermal and Adiabatic Changes:
|
Isothermal Changes |
Adiabatic Changes |
|
Temperature (T) remains constant, i.e., ∆T = 0. |
Heat content (Q) remains constant, i.e.,
|
|
System is thermally
conducting to the surroundings. |
System is thermally
insulated from the surroundings. |
|
The changes occurs
slowly. |
The changes occurs
suddenly. |
|
Internal energy (U)
remains constant, i.e., |
Internal energy changes,
i.e., |
|
Specific heat becomes
infinite. |
Specific heat becomes
zero. |
|
Equation of isothermal
changes is P V = Constant |
Equation of adiabatic
changes is
|
|
Slope of isothermal
curve,
|
Slope of adiabatic
curve,
|
|
Coefficient of
isothermal elasticity; Ei
= P |
Coefficient of adiabatic
elasticity; Ea=
γ P |
Isothermal
Process:
What is
Isothermal Process?
A
process in which the temperature remains constant is called an isothermal
process. From the starting of the process till the end, the temperature remains
constant.
For the
process we have the equation as:
PV
= Constant
If a system undergoes changes from the state
A to B, considering an isothermal process, the temperature remains constant,
i.e., in isothermal surface then,
|
A - - - - - - - - - - - - isothermal
process - - - - -- - -> B
(P1, V1, T) (P2, V2,
T) |
Hence P1V1 = P2V2 =
nRT

Isothermal Process
Isothermal Process Examples:
There are various examples of processes where the
temperature of the system, some of them are:
·
Evaporation is
also an example of isothermal process.
·
Condensation is an
example of isothermal process.
·
All the reactions
going on in the refrigerator are isothermal as a constant temperature is
maintained in it.
·
The melting of ice
at zero degree is an example of isothermal process.
·
The reaction in a
heat pump is an example of isothermal process.
·
The boiling of
water at hundred degrees is an example of isothermal process.
Condition for Isothermal Process:
·
The walls of the container must be perfectly conducting.
·
The speed of process should be very slow.
Equation of Isothermal Process:
The ideal gas equation for n moles of a gas is
PV = n R ------ (1)
For a fixed mass (n fixed) of a gas undergoing an
isothermal process (T fixed), the above equation and in all isothermal process
Boyle’s law is obeyed.
Hence equation of state is
PV = constant ------ (2)
This equation is the “equation of state” of an
isothermal process.
Indication Diagram:
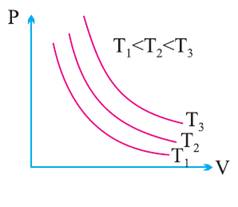
Done Work in
an Isothermal Process:
The pressure of a given mass of gas varies inversely
as its volume. This is Boyle’s Law. Suppose an ideal gas goes isothermally (at
temperature T) from its initial state (P1, V1) to the final state (P2, V2). At any intermediate
stage with pressure P and volume change from V to V + ![]() V (
V (![]() V small)
V small)
![]() W = P V
W = P V
Taking (![]() V <0) and summing the quantity
V <0) and summing the quantity ![]() W over the entire process,
W over the entire process,
W = ![]()
= µ RT![]()
∴ Work done = ![]() µ RT ln
µ RT ln![]()
Where in the second step we have made use of the ideal
gas equation PV = ![]() RT and taken the constants
out of the integral. For an ideal gas, internal energy depends only on
temperature. Thus, there is no change in the internal energy of an ideal gas in
an isothermal process. The First Law of Thermodynamics then implies that heat
supplied to the gas equals the work done by the gas: Q = W.
RT and taken the constants
out of the integral. For an ideal gas, internal energy depends only on
temperature. Thus, there is no change in the internal energy of an ideal gas in
an isothermal process. The First Law of Thermodynamics then implies that heat
supplied to the gas equals the work done by the gas: Q = W.
![]() Q =
Q = ![]() U + ∆W
U + ∆W
Note from the
above equation that for V2 > V1, W > 0; and for V2 < V1, W < 0. That is, in an
isothermal expansion, the gas absorbs heat and does work while in an isothermal
compression, work is done on the gas by the environment and heat is released.
First Law of Thermodynamics Applied to Isothermal
Process:
Applying
first law of thermodynamics to an isothermal process
![]() Q =
Q = ![]()
or
![]() =
= ![]()
·
When a gas expands isothermically:
An amount of heat equivalent
to the work done by the gas has to be supplied from an external source. ![]() &
& ![]() are positive and so
are positive and so ![]() will also be positive.
will also be positive.
·
When a gas compressed isothermically:
An amount of heat equivalent
to the work done on the gas has to be removed from the gas.
·
In an isothermal compression or expansion, the internal energy of the
gas remains unchanged.
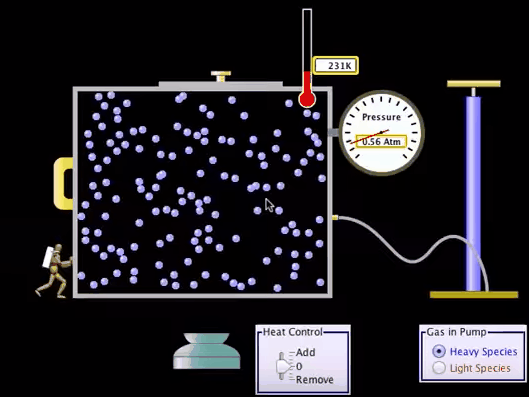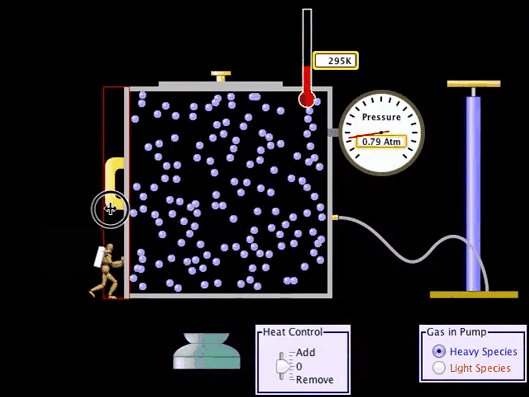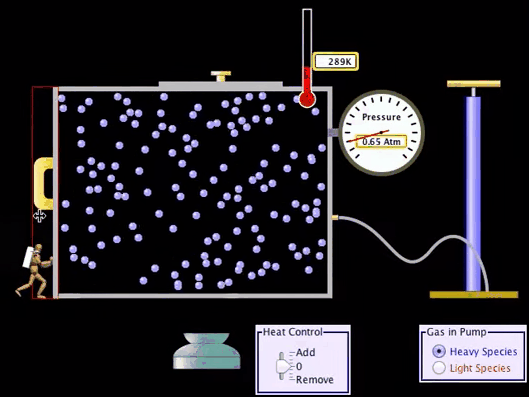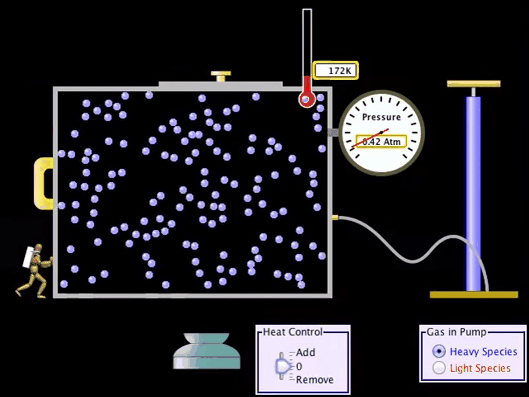
Adiabatic Process:
What is Adiabatic Process?
An adiabatic process
is a Thermodynamic process in which there is no heat or matter transfer into or
out of a system and is generally obtained by surrounding the entire system with
a strongly insulating material or by carrying out the process so quickly that
there is no time for a significant heat or matter transfer to take place.
Adiabatic Process
Adiabatic Processes:
·
Adiabatic is a
process in which there is no heat flow takes place between the system and the
surroundings.
·
These processes
are sudden.
·
The walls of the
container should be adiabatic.
·
For an adiabatic
process of an ideal gas.
From Boyle’s law,
|
PV γ = constant |
Where γ = ![]() Specific heat ratio.
Specific heat ratio.
Example: Hot tea in Thermos flask. It will remain hot
as there is no exchange of heat takes place because the walls of thermos is
insulating.
Thermal flask
Graphically:
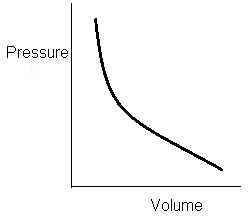
As pressure and volume are inversely proportional we will have decreasing
curve. This curve is known as Adiabatic Curve.
Condition for Adiabatic Process:
·
All the walls of
the container and the piston must be perfectly insulating.
·
The speed of
process should be fast.
Example of Some Adiabatic Process:
·
Sudden bursting of
the tube of bicycle tyre.
·
Propagation of
sound waves in air and other gases.
Energy in Adiabatic Process:
For adiabatic process, ΔQ = 0,
∴ ΔU + ΔW = 0
·
If ΔW = positive then ΔU = negative i.e., adiabatic
expansion produce cooling.
·
If ΔW = negative then ΔU = positive i.e., adiabatic
compression produce heating.
Adiabatic Change
of an Ideal Gas:
It implies how much work is done during adiabatic change of an ideal gas.
Initially ideal gas is at Pressure P1, Volume V1 and
Temperature T1 (P1, V1, T1).
Final state of an ideal gas Pressure P2, Volume V2 and
Temperature T2 (P2, V2, T2)
P
V γ = constant
γ = ![]()
If an ideal gas undergoes a change
in its state adiabatically from (P1, V1) to (P2,
V2)
P1V1 γ = P2V2 γ
The work done in an adiabatic change of an ideal gas from the state (P1,
V1, T1) to the state (P2, V2,
T2).
W = ∫
P V Dv
= P∫V dV (Integrating between the limits V2 and
V1)
For Adiabatic Process:
P
V γ = constant and this implies P= ![]()
W = constant ∫![]()
= constant ![]()
= ![]()
= 
By solving,
Work done W = ![]()
where,
T2 = final Temperature
T1 = initial temperature
R = Universal gas constant
γ = Specific heat ratio
This is the work done during adiabatic change.
Consider:
W = ![]()
Case 1: W>0
(when T1>T2)
Temperature of the gas decreases.
Case 2: W< 0 (T1<T2)
Temperature of the gas increases.
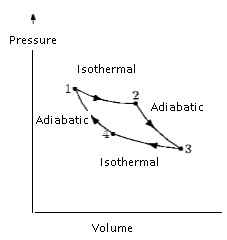
P-V curves for
isothermal and adiabatic processes of an ideal gas.
Equation of State:
·
Adiabatic relation between P and V: PVγ = K
·
Adiabatic relation between P and T: P1-γTγ = K
·
Adiabatic relation between T and V: TVγ-1 = K
Indication Diagram:
Slope of adiabatic curve: tan ϕ
= -γ![]()
Cyclic and Non-cyclic Process:
A cyclic process consists
of a series of changes which return the system back to its initial state. In
non-cyclic process the series of changes involved do not return the system back
to its initial state.
1.
In
cyclic process change in internal energy is zero and temperature of system
remains constant.
2.
Heat
supplied is equal to the work done by the system.
3.
For
cyclic process P–V graph is a closed curve and area enclosed by the closed path
represents the work done.
If the cycle is clockwise
work done is positive and if the cycle is anticlockwise work done is negative.
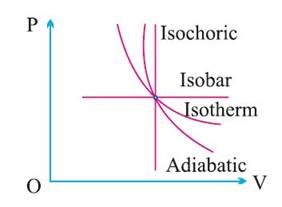
Graphical Representation of Various Processes
Heat engine is a device
which converts heat into work continuously through a cyclic process.
The essential parts of a
heat engine are:
Source: Working substance: Steam, petrol etc.
Sink: ‘‘efficiency’’ η
is given by
η = 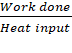
=![]()
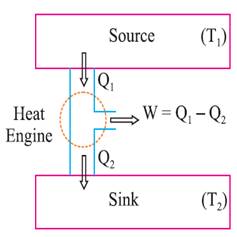
A perfect heat engine η = 1. Practically efficiency is always less than 1
Work Done in an Adiabatic Process:
Consider a unit mole of gas contained in a perfectly
non-conducting cylinder provided with a non-conducting and frictionless piston.
Let Cv be the specific heat of
gas at constant volume. Let at any instant, when the pressure of gas is P, the gas be compressed by small volume
dV. Then work done on the gas is,
dW = PdV
Total work done on gas to compress from volume v1 to v2
is given by
![]() = W =
= W = ![]() ------
(1)
------
(1)
According to first law of thermodynamics,
dQ = dU![]() PdV
PdV
For adiabatic process, dQ = 0
−dU
= PdV = −CvdT
W = ![]()
= ![]()
where,
T1 is the temperature
of gas when volume is V1 and T2 when volume
is V2.
Thus, work done is given by,
W = ![]()
=![]() − Cv(T2-T1)
− Cv(T2-T1)
= Cv(T1-T2)
Wadi =![]()
The above expression gives us the amount of work done in adiabatic process.