Second Law of Thermodynamics
What is Second Law of
Thermodynamics?
·
First
law of thermodynamics states the equivalence of heat and energy. It does not
state anything about the limitation in the conversion of heat into work or
about the condition necessary for such conversion.
·
Second
law of thermodynamics is generalization of certain experience and observation
and is concerned with tine direction in which energy flow takes place.
·
This
law can be stated in number of ways. Although differently said, they are
essentially equivalent.
(i) Kelvin Plank
Statement:
"It is impossible to construct a
device which, operating in a cycle, has a sole effect of extracting heat from a
reservoir and performing an equivalent amount of work".
(ii) Clasius
Statement:
"It is impossible for a self acting machine, unaided by internal agency, to
transfer heat from a colder body to a hotter body".
·
It
can be proved that these two statements of second law are completely equivalent
and violation of Kelvin Plank statement leads to violation of Clasius statement and vice-versa.

Second Law of Thermodynamics
Significance of second law of
thermodynamics:
·
The second
law puts a limits to the efficiency of heat engine and the co efficient of
performance of a refrigerator.
·
According to
second law,
·
The
efficiency of a heat engine can never be unity which implies that the heat
released to the cold reservoir can never be made zero.
·
The co
efficient of performance of a refrigerator can never be infinite which implies
that the external work (W) can never be made zero.
Limitations of second law of
thermodynamics:
·
The second
law of thermodynamics cannot be proven directly. But its validity has not been
contradicted by any machine designed so far.
·
It is
applicable to a cyclic process in which the system returns to its original
state after a complete cycle of changes.
·
It makes no
predictions as to what will happen under certain conditions but simply states
what will happen under a given set of conditions.
Reversible and irreversible process:
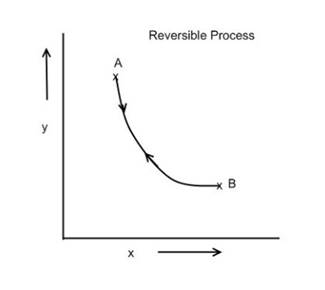
What is a Reversible Process?
The process in which the system and surroundings can be restored
to the initial state from the final state without producing any changes in the
thermodynamics properties of the universe is called a reversible process. In
the figure below, let us suppose that the system has undergone a change from
state A to state B. If the system can be restored from state B to state A, and
there is no change in the universe, then the process is said to be a reversible
process. The reversible process can be reversed completely and there is no
trace left to show that the system had undergone thermodynamic change undergo
reversible change, it should occur infinitely slowly due to infinitesimal
gradient. During reversible process all the changes in state that occur in the
system are in thermodynamic equilibrium with each other.
Thus there are two important conditions for the reversible process
to occur. Firstly, the process should occur in infinitesimally small time and
secondly all of the initial and final state of the system should be in
equilibrium with each other.
If during the reversible process the heat content of the system
remains constant, i.e. it is adiabatic process, then the process is also
isentropic process, i.e. the entropy of the system remains constant.
The phenomenon of undergoing reversible change is also called
reversibility. In actual practice the reversible process never occurs, thus it
is an ideal or hypothetical process.
Conditions for Reversible Process:
There
are two important conditions for the reversible process to occur.
·
Firstly,
the process should occur in infinitesimally small time.
·
Secondly,
all the initial and final state should be in the equilibrium with each other.
Reversibility in Thermodynamics:
The phenomena of undergoing reversible change is
also called reversibility. In actual practice the reversible process never
occurs, thus it is an ideal or hypothetical process.
Reversible Process Example:
Although no actual change is completely reversible
by the process of liquification and evaporation of a
system performed slowly are practically reversible. Similarly slow compression
of the gas in a cylinder is reversible process as gas can be expand slowly by
decreasing the weight on the piston to reverse the operation.
What is an Irreversible Process?
The irreversible process is also called the natural process
because all the processes occurring in nature are irreversible processes. The
natural process occurs due to the finite gradient between the two states of the
system. For instance, heat flow between two bodies occurs due to the
temperature gradient between the two bodies; this is in fact the natural flow
of heat. Similarly, water flows from high level to low level, current moves
from high potential to low potential, etc.
Here are
some important points about the irreversible process:
1) In
the irreversible process the initial state of the system and surroundings
cannot be restored from the final state.
2) During
the irreversible process the various states of the system on the path of change
from initial state to final state are not in equilibrium with each other.
3)
During the irreversible process the entropy of the system increases decisively
and it cannot be reduced back to its initial value.
4) The
phenomenon of a system undergoing irreversible process is called as
irreversibility.
Irreversible
process examples:
·
The
conduction of heat from a hot body to a cold body.
·
Production
of heat by the friction
·
Producing
of heat by the passing of current through an electrical resistance
·
Transfer
of heat by radiation
·
An
explosion
·
Inelastic
deformation
·
Magnetization
or polarization with a hysteresis
·
Spontaneous
chemical reactions
·
Spontaneous
mixing of matter of varying states
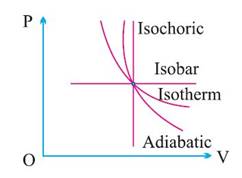
Cyclic and Non-cyclic Process:
A cyclic process consists
of a series of changes which return the system back to its initial state.
In non-cyclic process the
series of changes involved do not return the system back to its initial state.
(1) In
cyclic process change in internal energy is zero and temperature of system
remains constant.
(2)
Heat supplied is equal to the work done by the system.
(3)
For cyclic process P–V graph is a closed curve and area enclosed by the closed
path represents the work done.
If the cycle is clockwise
work done is positive and if the cycle is anticlockwise work done is negative.
Graphical Representation of Various Processes
Heat engine is a device
which converts heat into work continuously througha
cyclic process.
The essential parts of a
heat engine are :
Source : Working substance : Steam, petrol etc.
Sink : ‘‘efficiency’’ η
is given by

![]()
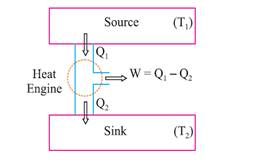
A perfect heat engine η = 1. Practically efficiency is always less than 1.