Heat Engine and Heat Pumps
Heat
Engine:
What
is Heat Engine?
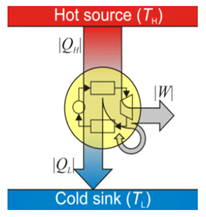
Heat engine is a device by which a system is made to undergo a
cyclic process that results in conversion of heat to work. It consists of a
working substance called the system.
Example: A mixture
of fuel vapour and air in a gasoline or diesel engine
or steam in a steam engine are the working substances.
Working of Heat Engine:
The working substance goes through a cycle consisting of
several processes. In some of these processes, it absorbs a total amount of
heat Q1 from an external
reservoir at some high temperature T1.
In some other processes of the cycle, the working substance releases a total
amount of heat Q2 to an
external reservoir at some lower temperature T2. The work done (W)
by the system in a cycle is transferred to the environment via some arrangement
(e.g. the working substance may be in a cylinder with a moving piston that
transfers mechanical energy to the wheels of a vehicle via a shaft). The cycle
is repeated again and again to get useful work for some purpose. The discipline
of thermodynamics has its roots in the study of heat engines.
Heat Engine
The efficiency (h) of a heat engine is defined by,
h = ![]() ------
(1)
------
(1)
Where Q1 is the
heat input i.e., the heat absorbed by the system in one complete cycle and W is the work done on the environment in
a cycle. In a cycle, a certain amount of heat (Q2) may also be rejected to the environment. Then,
according to the First Law of Thermodynamics, over one complete cycle,
W = Q1
– Q2 ------ (2)
i.e.,
h = 1 − ![]() ------
(3)
------
(3)
For Q2 = 0, h = 1, i.e., the
engine will have 100% efficiency in converting heat into work.
Note that the First Law of Thermodynamics i.e., the energy conservation law does not
rule out such an engine. But experience shows that such an ideal engine
with h = 1 is never possible, even if we can eliminate various kinds of losses
associated with actual heat engines. It turns out that there is a fundamental
limit on the efficiency of a heat engine set by an independent principle of
nature, called the Second Law of Thermodynamics. The mechanism of conversion of
heat into work varies for different heat engines. There are two ways: the
system (say a gas or a mixture of gases) is heated by an external furnace, as
in a steam engine; or it is heated internally by an exothermic chemical
reaction as in an internal combustion engine. The various steps involved in a
cycle also differ from one engine to another.
Types of Heat Engine:
Steam engine:
•
Internal combustion engine
•
Gas turbine
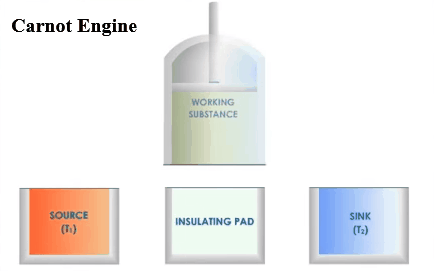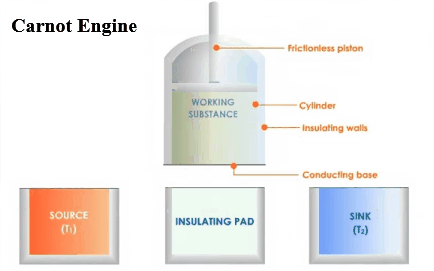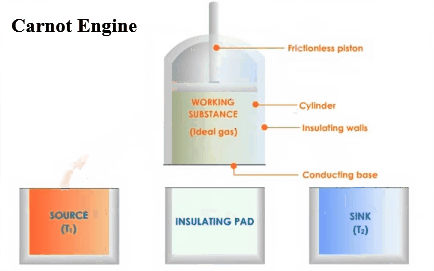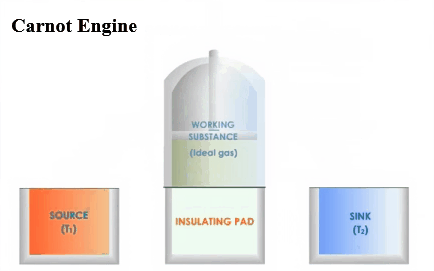
There are three main parts in an
engine. A hot body called source,
a working substance, and a body
called sink. There must be the source
of heat of infinite thermal capacity, and that should be at a constant high
temperature so that if any amount of heat is withdrawn from it or given to
it, that does not affect its temperature. It
must be some substance through which the heat is absorbed or rejected into the
sink .This is the working substance. There must be a sink of finite thermal
capacity, and that should be at a constant high temperature so that if any
amount of heat is withdrawn from it or given to it, that does not affect
its temperature.
Carnot
Engine:
Carnot designed a theoretical engine. This
engine cannot be realized in actual practice.
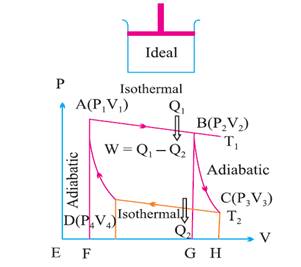
Carnot cycle:
The working substance of the engine undergoes a
cycle known as Carnot cycle. It consists of the following four strokes.
I.
Isothermal expansion.
II.
Adiabatic expansion.
III.
Isothermal compression.
IV.
Adiabatic compression.
Carnot Engine
Efficiency
of Carnot Cycle:
η = ![]()
= ![]()
η =1− ![]()
Where T1 and T2 are
in Kelvin.
1.
Efficiency of a heat engine depends
only on temperatures.
2.
Efficiency of a heat engine is always
lesser than unity, i.e.,
whole of heat can never be converted
into work which is in accordance with second law.
Carnot Theorem:
Carnot’s reversible
engine working between two given temperature is considered to be the most
efficient engine.
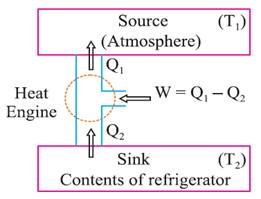
Refrigerator
and Heat pump:
A refrigerator is the reverse of a heat engine. Here the working
substance extracts heat Q2
from the cold reservoir at temperature T2,
some external work W is done on it
and heat Q1 is released to
the hot reservoir at temperature T1.
Example: Most commonly used refrigerant in heat
pump is Chlorofluorocarbons
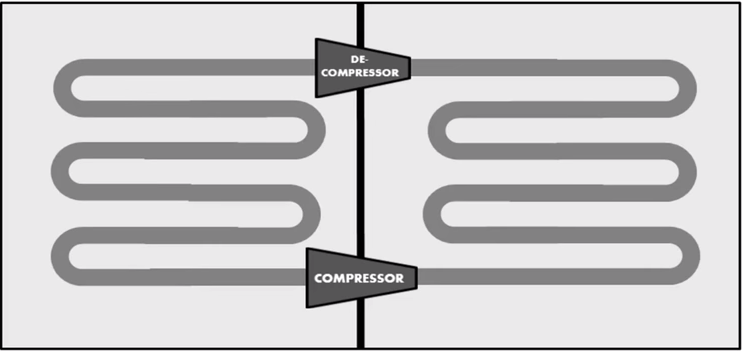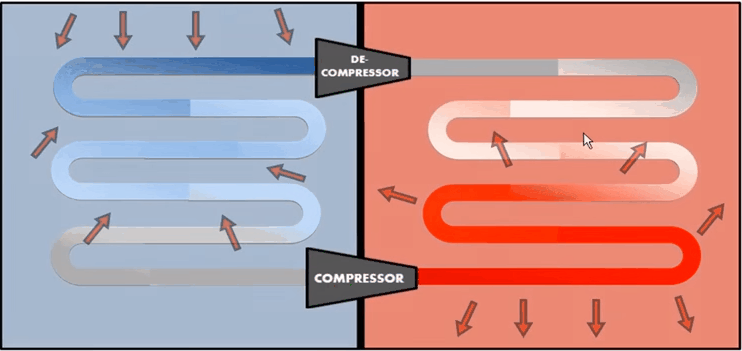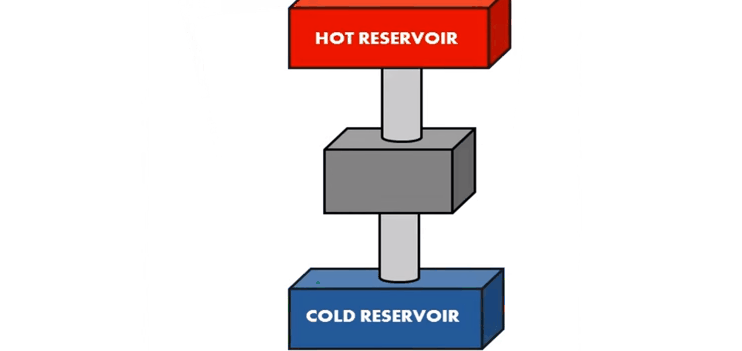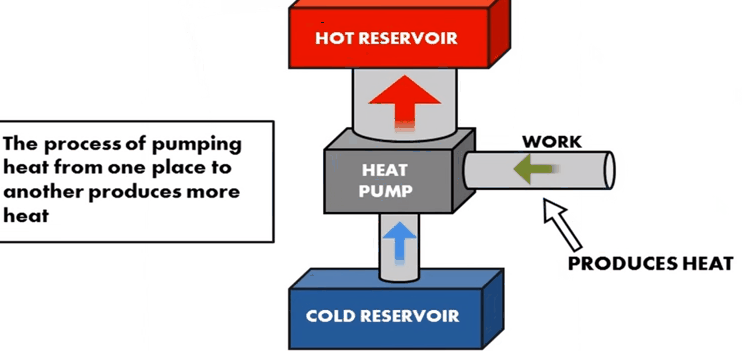
Compressor and Decompressor
A heat pump is the same as a refrigerator. If the purpose is to
cool a portion of space, like the inside of a chamber, and higher temperature
reservoir is surrounding the device, a refrigerator, if the idea is to pump
heat into a portion of space (the room in a building when the outside
environment is cold), the device is called a heat pump.
In a
refrigerator the working substance (usually, in gaseous form) goes through the
following steps:
1.
Sudden
expansion of the gas from high to low pressure which cools it and converts it
into a vapour-liquid mixture,
2.
Absorption
by the cold fluid of heat from the region to be cooled converting it into vapour,
3.
Heating
up of the vapour due to external work done on the
system,
4.
Release
of heat by the vapour to the surroundings, bringing
it to the initial state and completing the cycle.
The
coefficient of performance (a) of a refrigerator is given by
a = ![]() ------ (1)
------ (1)
Where
Q2 is the heat extracted
from the cold reservoir and W is the
work done on the system–the refrigerant. (a for heat
pump is defined as ![]() )
)
Note
that while h by definition can never exceed 1, a can be greater than 1. By
energy conservation, the heat released to the hot reservoir is,
Q1 = W + Q2
i.e.,
a = ![]() ------ (2)
------ (2)
In a
heat engine, heat cannot be fully converted to work; likewise a refrigerator
cannot work without some external work done on the system, i.e., the coefficient of performance
in Eq. (1) cannot be infinite.
Carnot Refrigerator:
·
For Carnot
refrigerator =![]() =
= ![]()
·
Coefficient of performance β = ![]()
where
![]() =
temperature of surrounding
=
temperature of surrounding
![]() =
temperature of cold body
=
temperature of cold body
Relation between coefficient of performance
and efficiency of refrigerator is,
|
β
= |
Purpose
and Advantage of Heat Pump:
•
The purpose of a heat pump is to transfer energy to a
warm environment, such as a home in the winter.
•
The great advantage of using a heat pump to keep your
home warm rather than just burning fuel in a fireplace or furnace is that a
heat pump supplies. It runs on electricity, so you can save substantially
on fuel consumption.
•
Heat pump warms air from one place to another, to where
it is needed depending on the season.
•
Even in the air that seems too cold, heat energy is
present.
•
When it’s cold outside, a heat pump extracts this
outside heat and transfers it inside.
•
When it’s warm outside, it reverses directions and acts
like an air conditioner, removing heat from your home.
Disadvantage
of Heat Pump:
The disadvantage of a heat pump is that the work input
is sometimes more expensive than simply burning fuel, especially if the work is
provided by electrical energy.