Dielectrics and Electric
Polarization
Dielectrics
Dielectrics
are non-conducting substances. In contrast to conductors, they have no or the
negligible number of charge carriers. In a dielectric, free movement of charges
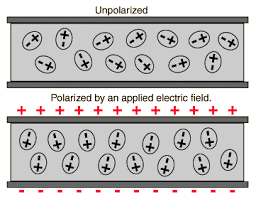
is not possible. It turns out that the external field induces dipole moment by
stretching or re-orienting molecules of the dielectric. The collective effect
of all the molecular dipole moments is net charges on the surface of the
dielectric which produce a field that opposes the external field.
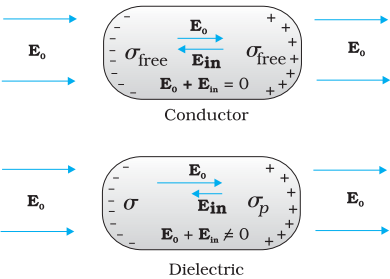
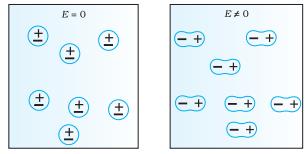
The difference in behaviour of a conductor and a
dielectric in an external electric field.
Types of dielectrics
Dielectrics can
be classified as:
·
Polar
Molecules
·
Non-Polar
Molecules
Polar
Molecules:
Polar Molecules are those type of dielectric
in which the possibilities that the positive and negative molecules will
coincide with each other is null or zero. In other words, When the
centre of positive and negative charges do not coincide because of the asymmetric
shape of the molecules, e.g., NH3, HCl,
etc.
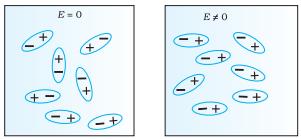
Non-Polar Molecule:
Unlike polar molecules in
non-polar molecules the center of positive charge and negative coincide, that
is it is not zero. The molecule then has
no permanent (or intrinsic) dipole moment. When the centre of positive charge
coincides with the centre of negative charge in a molecule, e.g., Nitrogen,
Oxygen, CO2 etc.
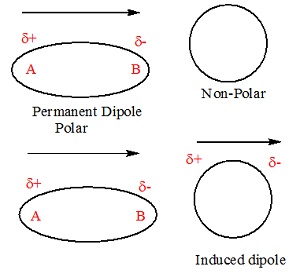
The non-polar molecule thus develops an induced dipole
moment. The dielectric is said to be polarized by the external field. Substances
for which this assumption is true are called linear isotropic dielectrics. The
induced dipole moments of different molecules add up giving a net dipole moment
of the dielectric in the presence of the external field.
The non-polar molecule thus develops an induced dipole
moment. The dielectric is said to be polarized by the external field. Substances
for which this assumption is true are called linear isotropic dielectrics. The
induced dipole moments of different molecules add up giving a net dipole moment
of the dielectric in the presence of the external field.
Induced Electric
Dipole Moment
When in a non-polar molecule, all
the protons are pulled in the direction as of electric field and electrons are
pulled in opposite direction as of electric field, when an external electric
field is applied. Due to the presence of electric field, this process continues
unless the internal forces balance them. Due to this two centers of charge are
created; the molecules are known as Polarized and is known
as Induced Electric Dipole. The dipole moment is
known as Induced Electric Dipole Moment.
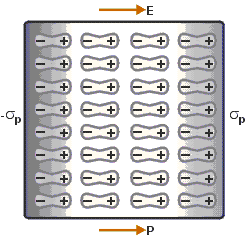
Electric Polarisation
When a dielectric slab is placed
in an electric field, then the dipole moment is gained by the molecule and the
dielectric is said to be polarised.
The Electric
Polarization is dipole moment per unit volume of a
dielectric material.
The polarization is denoted by P.
Polarization
Polarization
process
Dielectric Constant
When Dielectric slab is placed
between parallel plate, the ratio of the applied
electric field strength to the strength of the reduced value of electric field
capacitor is called Dielectric Constant that
is:
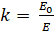
E is always
less than or equal to E.
where Eo is
dielectric and E is
net field
The larger the
dielectric constant, the more charge can be stored. Completely filling the
space between capacitor plates with a dielectric increases the capacitance by a
factor of the dielectric constant:
C = κ Co
where Co is the capacitance with
no dielectric between the plates.
Dielectric Polarization
Dielectric polarization
occurs when a dipole moment is formed in an insulating material because of
an externally applied electric field. When a current interacts with a
dielectric (insulating) material, the dielectric material will respond with a
shift in charge distribution with the positive charges aligning with the
electric field and the negative charges aligning against it.
By taking advantage of this response, important circuit elements such
as capacitors can be made.
Susceptibility
The electric
susceptibility can be defined as the ratio of Polarisation P to electric
field strength E,
![]() =
= ![]() =
= ![]()
where ϵ is the electric
permittivity
In MKS, the electric susceptibility is
defined as:
![]() =
= ![]() =
= ![]() – 1,
– 1,
where ϵ0 is the permittivity of free space
Permittivity
How much a
medium can be polarized in response to an applied electric field, this can
determine permittivity.
Units of
permittivity:
·
In
CGS units, ε is unit less.
·
In
SI units, ε is in units of Farads/meter.
In SI units,
ε0 is the permittivity of free space and has the value
ε0 ≈ 1.85 × 10-12 Farads/meter.
The
permittivity is given by:
![]() ( 1+
( 1+![]() ) SI
) SI
![]()