Cell
A cell
is a device that maintains the potential difference which exists between the
two electrodes due to chemical reaction. A collection of two or more cells
which are connected in series or parallel is called a Battery. Thus we will
obtain the required voltage or current. A battery is an energy source that
converts chemical energy to electrical energy. It is otherwise known as
electrochemical cell. The energy is stored in the form of chemical energy
inside a battery. Batteries give us a convenient source of energy for
energizing devices without cables and wires. When it is connected to a circuit
it produces electrical energy.
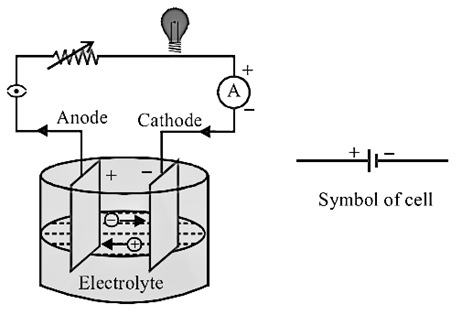
A
battery consists of two terminals – A Positive and Negative Terminal. The
Positive Terminal is called Cathode and the Negative Terminal is
called Anode. They are also called as Electrodes of a Cell. These
electrodes will be dipped in a solution called electrolyte. It is liquid which
is ionic and conducts electricity. The output voltage of battery depends upon
the elements used as electrodes, the size of the electrodes and the type of
electrolyte used in it.
When
the battery is about to charge, an external source is connected to it. The
anode of the battery is connected to the negative terminal of the source and
cathode is connected to the positive terminal of the source. As the external
source is connected to the battery, electrons are inserted into the anode. When
the cell or battery is connected to the circuit chemical reactions takes place.
Thus chemical reactions take place within the two electrodes. Here oxidation
and reduction reactions happen. Then reduction reaction occurs at cathode and
oxidation process occurs at anode.
The
cathode acts as the oxidizing agent by accepting electrons from the negative terminal
anode. The anode acts as the
reducing agent by losing the electrons. Thus due to these chemical reactions an
electrical difference occurs between the terminals-anode and cathode. When
there is no power the electrolyte prohibits the movement of electrons directly
from anode to cathode. This is why we are using an external source or
connecting to a circuit. Thus electrons travel from anode to cathode when the
circuit is closed. Finally it gives power to the appliance which is connected
to it. After a long time when the electrochemical process alters the anode and
cathode materials it stop giving out electrons. Then the battery dies.
Emf of cell (E):
EMF or
Electromotive force is defined as the potential difference which is developed
between the two terminals of a battery in an open circuit. We know that anode
has positive potential (V+)
and cathode has negative potential (V-).
So emf is the potential difference between the
positive terminal anode and negative terminal cathode when there is no current flowing through it. The emf measures the energy which is transferred to the charge
carries in the cell or a battery. It is the energy in joules divided by the
charge in coulombs. The emf acts as the initiating
force for the current to flow.
ε = ![]()
where
ε is the
electromotive force
E is the
energy
Q is the
charge
The emf
which is denoted by ε and the equation is given by ε = V+ - (-V-)
= V+ + V-. It is measured in volts.
Potential
difference (V):
The
voltage across the terminals of a cell when it is supplying current to external
resistance is called potential difference or terminal voltage. Potential
difference is equal to the product of current and resistance of that given part
i.e. V = iR.
Internal
resistance (r):
In case
of a cell the opposition of electrolyte to the flow of current through it is
called internal resistance of the cell.
The
internal resistance of a cell depends on
Ø the
distance between electrodes
Ø area of
electrodes
Ø and
nature, concentration
Ø and
temperature of electrolyte
A cell is
said to be ideal, if it has zero internal resistance.
Cell in
Various Positions
Closed
circuit:
Cell
supplies a constant current in the circuit.
(i) Current given by the cell ![]()
(ii) Potential difference across the resistance ![]()
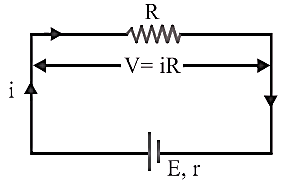
(iii) Potential drop inside the cell ![]()
(iv) Equation of
cell ![]()
(v) Internal resistance of the cell ![]()
(vi) Power
dissipated in external resistance (load)
![]()
Power delivered will be maximum when ![]()
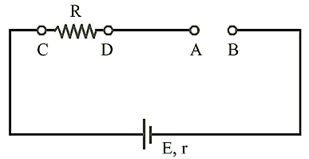
Open circuit:
When no current is taken from
the cell it is said to be in open circuit.
(i) Current through the circuit
i = 0
(ii) Potential difference
between A and B, ![]()
(iii) Potential difference
between C and D, ![]()
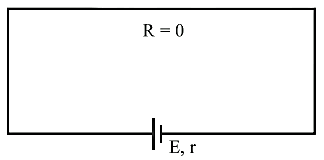
Short
circuit:
If two
terminals of cell are join together by a thick conducting wire
(i) Maximum current (called
short circuit current) flows momentarily ![]()
(ii) Potential difference V = 0
Important
1. It is important to note that
during charging of a cell, the positive electrode of the cell is connected to
positive terminal of battery charger and negative electrodes of the cell is
connected to negative terminal of battery charger. In this process, current
flows from positive electrode to negative electrode through the cell.
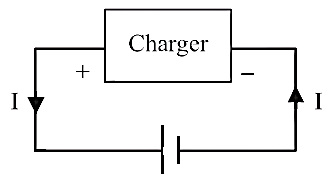
Therefore, V = ε + Ir
Hence, the terminal potential
difference becomes greater than the emf of the cell.
2. The difference of emf and terminal voltage is called lost voltage as it is
not indicated by a voltmeter. It is equal to Ir.
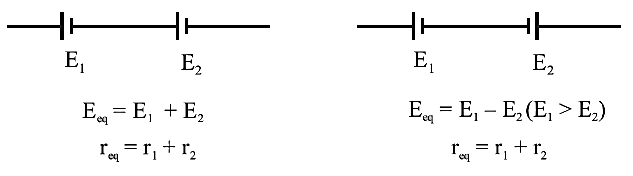
Grouping of Cells
In
series grouping of cell’s their emf’s are additive or
subtractive while their internal resistances are always additive. If dissimilar
plates of cells are connected together their emf’s are added to each other while if their similar
plates are connected together their emf’s are
subtractive.
I=
(nE)/(R+nr)
If
R<<nr, then I = E/R
If
R>>nr, then I = nE/R
Series grouping:
In
series grouping anode of one cell is connected to cathode of other cell and so
on. If n identical
cells are connected in series:
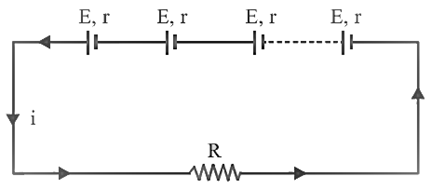
·
Equivalent emf of the
combination ![]()
·
Equivalent internal resistance ![]()
·
Main current = Current from each cell ![]()
·
Potential difference across external resistance ![]()
Parallel
grouping:
In parallel
grouping all anodes are connected at one point and all cathode are connected
together at other point. If n identical cells are connected in parallel:
I= E/[R+(r/m)]
If
R>>r/m, then I = E/R
If
R>>r/m, then I = m(E/R)
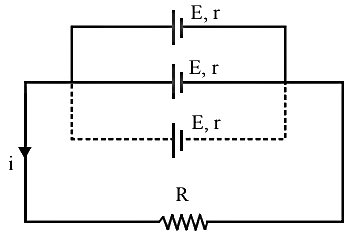
·
Equivalent emf of the
combination ![]()
·
Equivalent internal resistance ![]()
·
Main current = Current from each cell ![]()
·
Potential difference across external resistance ![]()
Generalized
Parallel Battery
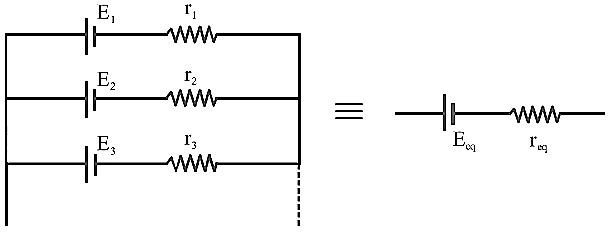
![]()
![]()
Mixed
Grouping:
If n identical cell’s are connected in a row and
such m row’s are connected in parallel as shown.
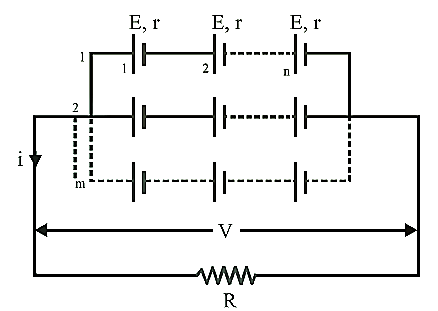
·
Equivalent emf of the
combination ![]()
·
Equivalent internal resistance ![]()
·
Main current = Current from each cell ![]()
·
Potential difference across external resistance ![]()
Mixed
grouping:-
(a)
I = mnE/(mR+nr)
(b)
I is maximum when nR = mR
(c)
Imax = mnE/(2√mnrR)